of Influenza Virus NS1 to the Inhibition of Type I Interferon
Production and Activation of Human Dendritic Cells
Irene Ramos,aElena Carnero,a* Dabeiba Bernal-Rubio,aChristopher W. Seibert,a,bLiset Westera,a* Adolfo García-Sastre,a,c,d Ana Fernandez-Sesmac,d,a
Department of Microbiology,aGraduate School of Biological Sciences,bGlobal Health and Emerging Pathogens Institute,cand Department of Medicine,dDivision of
Infectious Diseases, Mount Sinai School of Medicine, New York, New York, USA
The influenza virus nonstructural protein 1 (NS1) inhibits innate immunity by multiple mechanisms. We previously reported that NS1 is able to inhibit the production of type I interferon (IFN) and proinflammatory cytokines in human primary dendritic cells (DCs). Here, we used recombinant viruses expressing mutant NS1 from the A/Texas/36/91 and A/Puerto Rico/08/34 strains in order to analyze the contribution of different NS1 domains to its antagonist functions. We show that the polyadenylation stimulating factor 30 (CPSF30) binding function of the NS1 protein from A/Texas/36/91 influenza virus, which is absent in the A/Puerto Rico/08/34 strain, is essential for counteracting these innate immune events in DCs. However, the double-stranded RNA (dsRNA) binding domain, present in both strains, specifically inhibits the induction of type I IFN genes in infected DCs, while it is essential only for inhibition of type I IFN proteins and proinflammatory cytokine production in cells infected with influenza viruses lacking a functional CPSF30 binding domain, such as A/Puerto Rico/08/34.
T
he influenza A virus (IAV) nonstructural protein 1 (NS1) has multiple functions that allow the virus to replicate efficiently and to antagonize host immune responses (reviewed in reference 1). Several NS1 functions contributing to IAV virulence are re-lated to its double-stranded RNA (dsRNA) binding function and have been mapped to the N-terminal domain of the NS1 (2), and most of them are related to the ability of NS1 to counteract the host antiviral state by inhibiting type I interferon (IFN) produc-tion (reviewed in reference1). Several years ago, the influenza virus NS1 was shown to inhibit interferon regulatory factor 3 (IRF3) activation in infected cells (3). Also, the NS1 has the ability to prevent NF-B activation, which is necessary for the produc-tion of IFN-␣/in virus-infected cells (4). NS1 sequesters dsRNAs by interacting with them, thus avoiding recognition and activa-tion of the 2=,5=-oligo(A) synthetase (OAS)-RNase L pathway (5) and the type I IFN-induced protein kinase RNA activated (PKR) (6–8). The NS1 protein is also known to interact directly with PKR in a dsRNA-binding-independent manner, repressing its activity (9). Importantly, NS1 interacts with retinoic acid-inducible gene I (RIG-I) and tripartite motif protein 25 (TRIM25), avoiding the RIG-I-mediated induction of type I IFN (10,11). These NS1 prop-erties are particularly important for avoiding viral detection by cytosolic sensors, as well as for modulation of the antiviral re-sponses at the pretranscriptional level. Influenza NS1 is also able to exert an effect on the posttranscriptional processing of the host mRNA and the nuclear export of mRNAs which contain 3= -poly(A) ends (2,12). This function is localized to the C-terminal portion of the protein, or effector domain (2), and results primar-ily from the interaction of the influenza NS1 protein with poly-adenylation stimulating factor 30 (CPSF30), the 30-kDa subunit of cleavage (13), as well as with the poly(A)-binding protein II (PABII) (14). Thus, NS1 selectively inhibits 3=end processing of cellular pre-mRNAs. More recently, it was also shown that the C-terminal tail of the NS1 of human H3N2 influenza viruses acts as a histone tail mimic and inhibits host transcriptional elongationby interaction with the human RNA polymerase II-associated fac-tor (PAF) complex (15). In addition, NS1 preferentially enhances translation of the influenza virus mRNA by recruiting eukaryotic translation initiation factor 4GI (eIF4GI) specifically to its 5= un-translated region (16,17). The NS1 protein has also been involved in the regulation of the activation of phosphatidylinositol 3-kinase (PI3K) by binding the p85subunit, resulting in PI3K activation (18,19).
Although the main target cells for influenza viruses are respi-ratory epithelial cells, understanding the modulation of innate immune responses in human primary immune cells such as den-dritic cells (DCs) and macrophages is of great importance in order to learn how influenza virus might manipulate the human innate immune system and adaptive immunity, leading to enhanced vir-ulence. DCs are located in primary barriers of the body, such as mucosa and skin, and act as sentinels of the immune system. Upon sensing invading pathogens via several pattern recognition recep-tors (PRRs), they differentiate to mature DCs, producing high levels of IFN and proinflammatory cytokines, which are impor-tant in the establishment of the antiviral state in those cells and neighboring cells. Additionally, activated DCs migrate to the sec-ondary lymphoid organs, where the processed antigen is
pre-Received20 August 2012 Accepted10 December 2012
Published ahead of print19 December 2012
Address correspondence to Irene Ramos, irene.ramos-lopez@mssm.edu, or Ana Fernandez-Sesma, ana.sesma@mssm.edu.
* Present address: Elena Carnero, Center for Applied Medical Research, University of Navarra, Pamplona, Spain; Liset Westera, Department of Immunology, University Medical Center Utrecht, Utrecht, The Netherlands.
Copyright © 2013, American Society for Microbiology. All Rights Reserved.
doi:10.1128/JVI.02247-12
The authors have paid a fee to allow immediate free access to this article.
on November 7, 2019 by guest
http://jvi.asm.org/
sented to T lymphocytes (reviewed in reference20). Therefore, DCs are essential players in the induction of innate immunity, as well as in the initiation of adaptive responses.
We reported previously that influenza virus NS1 is able to counteract the production of type I IFN and the activation of human primary DCs (21–23). However, the mechanisms under-lying these inhibitory functions in infected DCs are not well un-derstood. Therefore, we investigated the contribution of different NS1 domains in order to obtain a better understanding of the antagonist role of NS1 in human primary DCs.
MATERIALS AND METHODS
Cells.Human primary DCs were generated from CD14⫹cells isolated from buffy coats of healthy human donors (New York Blood Center) as described previously (21,24). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation (His-topaque; Sigma-Aldrich), and CD14⫹cells were purified using CD14 an-tibody-labeled magnetic beads and MiniMACS LS columns (Miltenyi Biotech). CD14⫹cells were incubated at 37°C for 5 days at a concentration of 106 cells/ml in RPMI containing 10% fetal bovine serum (FBS)
(HyClone Thermo Scientific), 2 mML-glutamine, 1 mM sodium pyru-vate, 100 U/ml penicillin-100g/ml streptomycin (Gibco Invitrogen) (complete DC medium) and were supplemented with 500 U/ml human granulocyte-macrophage colony-stimulating factor (hGM-CSF) and 1,000 U/ml human interleukin 4 (hIL-4) (PeproTech).
MDCK and A549 cells were maintained in minimal essential medium (MEM) (Gibco Invitrogen) and Dulbecco’s minimum essential medium
(DMEM) (Gibco Invitrogen), respectively, supplemented with 10% FBS and penicillin-streptomycin (Gibco Invitrogen).
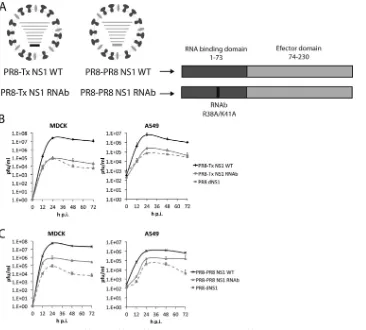
Recombinant viruses.Recombinant influenza viruses were generated using reverse genetics techniques as described previously (25). All the viruses contained the 7 genesPB2,PB1,PA,HA,NP,NA, andMfrom A/Puerto Rico/8/1934 (PR8) and theNS1segment from PR8 or A/Texas/ 36/91 (Tx) as either the wild type (WT) or carrying the mutations indi-cated inFig. 1and5. All viruses were grown in 7-day-old embryonated chicken eggs (Spafas, Charles River Laboratories), and the titers were de-termined by immunostaining on MDCK cells using the anti-M1/M2 an-tibody E10 (22) following standard procedures.
Growth curves of recombinant viruses in cell lines.To examine viral replication, confluent MDCK or A549 cells were infected at multiplicities of infection (MOIs) of 0.001 and 0.01, respectively. The cells were incu-bated at 37°C in DMEM containing 0.3% bovine albumin (MP Biomedi-cals) and 1 g/ml of tolylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Sigma). Supernatants were collected at selected time points postinfection (p.i.), and viral titers were determined in a stan-dard plaque assay on MDCK cells.
Infections of human DCs with the recombinant influenza viruses. Primary human DCs were infected with the recombinant influenza vi-ruses at the MOIs indicated below and in the figure legends, using serum-free DC medium for 45 min at 37°C as described previously (21). Then, DCs were plated in complete DC medium (10% FBS) at a concentration of 106cells/ml and incubated for the remaining time to the desired time
point at 37°C. Subsequently, cells were recovered by centrifugation for 10 min at 400⫻g, and cell pellets were lysed for RNA isolation or protein analysis by Western blotting, while the supernatants were tested for cyto-FIG 1Characterization of the recombinant PR8-Tx NS1 WT, PR8-Tx NS1 RNAb, PR8-PR8 NS1 WT, and PR8-PR8 NS1 RNAb viruses. (A) Scheme of the recombinant viruses and the mutations introduced. (B and C) Growth kinetics of the viruses in MDCK and A549 cells. Average titers⫾SD are indicated.
Influenza Virus NS1 Antagonist Functions in Human DCs
on November 7, 2019 by guest
http://jvi.asm.org/
[image:2.585.112.478.65.395.2]kine production by a multiplex enzyme-linked immunosorbent assay (ELISA).
RNA isolation.RNA from DCs was extracted using TRIzol (Invitro-gen) according to the manufacturer’s instructions. The concentration was evaluated in a NanoDrop spectrophotometer at 260 nm, and for most of the experiments, 500 ng of RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). When indicated, we used the iScript Select cDNA synthesis kit (Bio-Rad) and added random primers or oligo(dT) primers.
Quantitative reverse transcription-PCR.Evaluation of cytokine ex-pression and viral genes was carried out using iQ SYBR green supermix (Bio-Rad) according to the manufacturer’s instructions. The PCR tem-perature profile was 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, and 60°C for 30 s. The primers used for detection of the M RNA segment from PR8 influenza virus, IFN-, tumor necrosis factor alpha (TNF-␣), interleukin 6 (IL-6), rps11, and␣-tubulin were as described previously (24). For IFN-␣quantification, we used 5=-CTGAATGACTTGGAAGCC TG-3=(forward) and 5=-ATTTCTGCTCTGACAACCTC-3=(reverse), for NP segment RNA from PR8 influenza virus, we used 5=-TACCTGCTTC TCAGTTCAAG-3=(forward) and 5=-CAGCCTAATCAGACCAAATG-3= (reverse), and for the amplification of PR8 and Tx NS1, we used 5=-TTC ACCATTGCCTTCTCTTC-3=(forward) and 5=-CCCATTCTCATTACT GCTTC-3=(reverse). All the reactions were performed in duplicate. The efficiencies of the primers were evaluated and in all cases were confirmed to be approximately 100%. The relative mRNA expression level was cal-culated by the threshold cycle (⌬CT) method (26); we used the two house-keeping genes␣-tubulin and rps11 to normalize the results (27).
Quantification of cytokine in supernatants of infected DCs. Quan-tification of IFN-␣, TNF-␣, and IL-6 release in infected human DC super-natants was performed using the Milliplex multi-analyte profiling human cytokine/chemokine kit (Millipore) according to the manufacturer’s in-structions. Data were analyzed using the Milliplex Analyst software (Mil-lipore). An interferon bioassay was used to detect active type I IFN pro-duction as described previously (28). Briefly, Vero cells were incubated overnight (ON) with UV-exposed supernatants from infected DCs, either undiluted or serial 3-fold dilutions. Then, cells were infected with a re-combinant Newcastle disease virus (NDV) expressing green fluorescent protein (GFP) at an MOI of 1, and the relative fluorescence intensity was quantified with a microplate reader (BioTek). Images were acquired using an Olympus IX70 fluorescence microscope.
Western blot analysis.At 12 h p.i., cells were lysed using an NP-40 lysis buffer (150 mM NaCl, 1.0% NP-40, 50 mM Tris [pH 8.0]), and the total protein concentration was evaluated using a Bradford assay (Bio-Rad). The same amount of protein per sample was run using Laemmli loading buffer (Bio-Rad) in 4 to 20% SDS-polyacrylamide Mini-PROTEAN TGX precast gels (Bio-Rad) under reducing conditions and then transferred to a polyvinylidene difluoride (PVDF) Immobilon trans-fer membrane (Millipore). Membranes were blocked in PBS-T (phos-phate-buffered saline, 0.05% Tween 20) containing 5% fat-free milk, washed 3 times with PBS-T, and incubated ON at 4°C with the following antibodies in PBS-T-1% bovine serum albumin (BSA): mouse anti-hem-agglutinin (HA) PR8, mouse anti-M1 PR8, and rabbit anti-NP PR8 (22), rabbit anti 1-73NS1TX/98 anti-NS1 (29), rabbit anti-phospho-IRF3 (Ser396), and IRF3 (Cell Signaling Technology) and mouse anti--actin (Sigma-Aldrich). The blots were washed and incubated with the corre-sponding secondary antibody, horseradish peroxidase (HRP)-linked sheep anti-mouse IgG or donkey anti-rabbit (GE Healthcare). Antibody-protein complexes were detected using a Western Lighting chemilumi-nescence system (PerkinElmer). For quantification of the phosphorylated IRF3 (IRF3-P) and total IRF3 levels, the software ImageJ was used in order to determine the mean pixel intensity of the bands.
Confocal microscopy.For influenza NS1 localization analysis, we in-fected DCs at an MOI of 1 for 4 h p.i. in glass-bottom 12-well plates (MatTek Corporation) coated with poly-L-lysine (Sigma-Aldrich). Then,
cells were fixed with 4% paraformaldehyde, permeabilized with 0.1%
Tri-ton X-100, and blocked with 10% goat serum in PBS. Subsequently, cells were stained with the polyclonal anti-NS1 antibody pAb155 (30), the secondary Alexa-Fluor 488 F(ab=)2fragment of goat anti-rabbit IgG
(In-vitrogen), and 4=,6-diamidino-2-phenylindole (DAPI) (Sigma). Images were acquired with a Zeiss LSM 510 confocal microscope taking Z-stack images of approximately 5m centered in the midsection of the cell nuclei to detect NS1 nuclear localization.
Statistics.The results are presented as average values of replicates plus or minus the standard deviation (SD). Average values were compared by the Studentttest. Experiments with human DCs were performed on sam-ples from at least three independent donors, and one representative ex-periment is shown in every case.
RESULTS AND DISCUSSION
Characterization of recombinant influenza viruses expressing A/Texas/36/91 and Puerto Rico/08/34 NS1 with altered dsRNA binding function.We first studied the involvement of the NS1 dsRNA binding domain in inhibition of innate immunity by the influenza A virus. We generated recombinant viruses bearing the NS1 from the human isolate A/Texas/36/91 (Tx) or A/Puerto Rico/8/34 (PR8) in a PR8 backbone (Tx NS1 WT and PR8-PR8 NS1 WT) using reverse genetics. The same backbone (PR8-PR8) was used in all the viruses included in this study to avoid variabil-ity due to the influence of other gene segments. As depicted inFig. 1A, we introduced the mutations R38A and K41A in NS1, previ-ously shown to abrogate the dsRNA binding functions without affecting NS1 dimerization (31), and rescued mutant viruses PR8-Tx NS1 RNAb and PR8-PR8 NS1 RNAb. The R38A and K41A mutations also result in a loss of binding to TRIM25 and, subsequently, a loss of inhibition of RIG-I activation (10). Multi-cycle replication kinetics of the recombinant viruses were ana-lyzed in MDCK and A549 cells (Fig. 1BandC) and showed that the R38A and K41A mutations resulted in attenuation of the vi-ruses in those cell lines to levels similar to those in an NS1-deleted PR8 virus (PR8 dNS1).
Next, we evaluated virus replication and expression of viral proteins in human primary DCs after infection with these recom-binant viruses. As shown inFig. 2A, we did not observe major differences in the RNA expression of NP, M, and NS1 at 3, 6, or 12 h p.i. in DCs infected with viruses bearing the mutant Tx or PR8 NS1 (PR8-Tx NS1 RNAb or PR8-PR8 NS1 RNAb) compared to the viruses bearing the WT NS1 (PR8-Tx NS1 WT and PR8-PR8 NS1 WT, respectively). Similarly, comparable levels of expression of HA, NP, M1, and NS1 were detected by Western blot analyses at 12 h p.i. in cells infected with the viruses PR8-Tx NS1 WT or PR8-Tx NS1 RNAb (Fig. 2B). In the case of PR8 dNS1 virus, we observed levels of NP similar to those of the NS1-bearing viruses but lower expression of HA and M1, as previously observed for A/Texas/91 NS1 truncation mutants (22). In the case of the PR8 NS1-expressing viruses, we also observed similar expression levels of HA, NP, and NS1 in Western blot analyses of DCs infected with both viruses (Fig. 2B), while the expression levels of M1 were slightly lower in the PR8-PR8 NS1 RNAb- than in the PR8-PR8 NS1 WT-infected DCs. Overall, these results indicate that mutations in the dsRNA binding site of the NS1 do not result in reduced viral protein expression of these viruses in human DCs, with the exception of M1 expression in cells infected with PR8-PR8 NS1 RNAb.
Since the NS1 dsRNA binding site is known to overlap with the nuclear localization site 1 (NLS1) of this protein (32), we analyzed the subcellular localization of NS1 in DCs by confocal micros-copy. As shown inFig. 2C, high NS1 protein expression was
on November 7, 2019 by guest
http://jvi.asm.org/
served in the cytoplasm. However, nuclear distribution of this protein was also observed, with a similar pattern for PR8-Tx NS1 WT- and PR8-Tx NS1 RNAb-infected cells. The same results were obtained when PR8-PR8 NS1 WT and PR8-PR8 NS1 RNAb were compared, indicating that the mutations R38A and K41A did not modify the subcellular localization of NS1 in DCs. Therefore, we can exclude the possibility that the subsequent effects of these mutations in cellular responses are a consequence of a different distribution of the NS1 protein.
The dsRNA binding function of NS1 from A/Texas/36/91 and A/Puerto Rico/08/34 contributes differently to the inhibition of type I IFN production and DC activation.To analyze the profile of type I IFN induction in DCs upon infection with the NS1 mu-tant viruses, we first measured the expression of IFN-␣and IFN- by qRT-PCR. As shown inFig. 3A, significantly higher levels of IFN-␣and IFN-RNA expression were observed in the PR8-Tx NS1 RNAb-infected DCs than in PR8-Tx NS1 WT-infected DCs. Similarly, the levels of IFN-␣and IFN-mRNAs in PR8-PR8 NS1
FIG 2Characterization of the recombinant PR8-Tx NS1 WT, PR8-Tx NS1 RNAb, PR8-PR8 NS1 WT, and PR8-PR8 NS1 RNAb viruses in human DCs. (A) Analysis of the expression of the NP, M, and NS1 viral genes in infected DCs by qRT-PCR. Averages of replicates⫾SD are indicated. (B) Expression of viral HA, NP, M1, and NS1 protein levels and-actin in infected DCs at 12 h p.i. by Western blot analysis. (C) Immunofluorescence staining of the subcellular localization of influenza virus NS1 protein in infected DCs at an MOI of 1 by confocal microscopy. One representative donor of three independent experiments with different donors is shown.
Influenza Virus NS1 Antagonist Functions in Human DCs
on November 7, 2019 by guest
http://jvi.asm.org/
[image:4.585.136.451.63.548.2]RNAb-infected DCs were significantly increased over the PR8-PR8 NS1 WT-infected DCs. Accordingly, infection with PR8-PR8-Tx NS1 RNAb and PR8-PR8 NS1 RNAb promoted levels of IRF3 phosphorylation (IRF3-P) in DCs similar to those promoted by the PR8 dNS1 virus (Fig. 3B) at 4 h p.i., corroborating the data on type I IFN mRNA induction by these viruses (Fig. 3A).
Interestingly, when the release of IFN-␣in the cell culture supernatant was measured by ELISA, we observed low levels of
IFN-␣production in the cultures infected with the PR8-Tx NS1 RNAb, comparable to those in PR8-Tx NS1 WT-infected samples (Fig. 3C). On the contrary, DCs infected with the mu-tant PR8- or NS1-expressing virus (PR8-PR8 NS1 RNAb) showed elevated levels of IFN-␣ protein production, which correlated with the IFN-␣mRNA quantification and IRF3-P data (Fig. 3C). These data suggest a differential processing of IFN mRNAs in cells infected with the recombinant viruses
car-FIG 3Analysis of the type I IFN expression induced by the recombinant PR8-Tx NS1 WT, PR8-Tx NS1 RNAb, PR8-PR8 NS1 WT, and PR8-PR8 NS1 RNAb viruses in human DCs. (A) qRT-PCR analysis of IFN-␣and IFN-. Averages of replicates⫾SD are indicated. (B) Analysis of IRF3-P and IRF3 levels by Western blotting at 4 h p.i. and the IRF3-P/IRF3 ratios of the mean pixel intensities. (C) IFN-␣protein detection in supernatants of infected cells by multiplex ELISA. Averages of replicates⫾SD are indicated. (D) NDV-GFP IFN bioassay. Cells exposed to nondiluted supernatants (ND) and dilutions 2, 4, and 6 from a 3-fold dilution series. (E) NDV-GFP IFN bioassay. Quantification of the relative fluorescence intensity from dilution 4 (dilution factor, 34). One representative donor
of three independent experiments with different donors is shown. Statistically significant differences (Pⱕ0.05) were determined by the Studentttest and are indicated by asterisks.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:5.585.103.482.67.541.2]rying RNAb mutations in Tx NS1 compared to those carrying the PR8-PR8 NS1 RNAb mutant.
Active type I IFN levels were also evaluated using an NDV-GFP bioassay. When Vero cells were exposed to undiluted superna-tants from infected cells, no NDV replication was observed in any case (Fig. 3D). NDV is very sensitive to the presence of type IFN; therefore, as observed in this experiment, low concentrations of
IFN-␣/can completely abrogate NDV replication. After two se-rial dilutions (dilution factor, 32), some NDV-GFP replication could be detected in PR8-Tx NS1 WT, PR8-Tx NS1 RNAb, and PR8-PR8 NS1 WT. The clearest differences were observed in the 4th dilution (dilution factor, 34); quantification of the relative fluorescence intensity is shown inFig. 3E. The levels of type I IFN in supernatants of cells infected with PR8-Tx NS1 WT and
FIG 4Analysis of cytokine expression induced by the recombinant PR8-Tx NS1 WT, PR8-Tx NS1 RNAb, PR8-PR8 NS1 WT, and PR8-PR8 NS1 RNAb viruses in human DCs. (A) qRT-PCR analysis of TNF-␣and IL-6. (B) TNF-␣and IL-6 protein detection in supernatants of infected cells by multiplex ELISA. (C) qRT-PCR analysis of IFN-␣, IFN-, TNF-␣, and IL-6 in DCs infected with PR8-Tx NS1 WT and PR8-Tx NS1 RNAb at 6 h p.i. using random primers or oligo(dT) primers for the cDNA synthesis reaction. These data were normalized to the PR8-Tx NS1 WT-infected samples. One representative donor of at least three independent experiments with different donors is shown. Average results from replicates⫾SD are indicated. Statistically significant differences (Pⱕ0.05) were determined by the Studentttest and are indicated by asterisks.
Influenza Virus NS1 Antagonist Functions in Human DCs
on November 7, 2019 by guest
http://jvi.asm.org/
[image:6.585.135.448.66.558.2]PR8-Tx NS1 RNAb were similar, confirming the results obtained by multiplex ELISA. However, PR8-PR8 NS1 RNAb supernatants from infected DCs showed higher type I IFN levels than the ones from PR8-PR8 NS1 WT-infected DCs, indicating an important inhibitory role for dsRNA binding function to the production of active type I IFN for PR8 but not for Tx NS1.
Therefore, these data suggest a different contribution of the dsRNA binding domain of NS1 between the two different strains. While in both cases this domain seems to be important for the inhibition of IRF3 phosphorylation and type I IFN induction, as measured at the mRNA level, the virus with the mutant Tx NS1 is still able to inhibit type I IFN production, as observed at the pro-tein level.
The production of two proinflammatory cytokines character-istic of DC activation and driven by the activation of the NF-〉 transcription factor, TNF-␣, and IL-6 (33,34) were also analyzed after infection of DCs with the WT and mutant NS1-bearing vi-ruses. As shown inFig. 4AandB, we did not observe significant differences in TNF-␣or IL-6 expression by qRT-PCR or multiplex ELISA between the PR8-Tx NS1 WT- and PR8-Tx NS1 RNAb-infected DCs. On the contrary, we observed that PR8-PR8 NS1 RNAb induced elevated levels of both cytokines compared to the PR8-PR8 NS1 WT and to the recombinant viruses bearing Tx NS1. Consequently, these results suggest an important role for the
dsRNA binding domain of the NS1 from PR8 in the inhibition of DC activation but not the one of Tx NS1.
All together, these data indicate that the dsRNA binding do-main of A/Texas/91 NS1 is not necessary for the NS1-mediated inhibition of DC activation, as measured by the production of proinflammatory cytokines in those cells, but it is required for the inhibition of type I IFN mRNA production (detected by qRT-PCR using a mix of random primers and oligo(dT) primers for the cDNA synthesis) and not for the inhibition of type I IFN protein expression. This is consistent with the loss of inhibition of RIG-I activation, IRF3 phosphorylation, and IFN promoter activation, previously described for an NS1 that despite having a defective dsRNA binding function is still able to inhibit host mRNA pro-cessing and IFN protein expression (10,11).
Infection with PR8-Tx NS1 RNAb promoted levels of IRF3-P in DCs similar to those promoted by the PR8 dNS1 virus (Fig. 3B), corroborating the data on type I IFN mRNA induction by these viruses (Fig. 3A). However, other NS1 functions remained active in the mutant PR8-Tx NS1 RNAb virus and might have been re-sponsible for its effect on type I IFN secretion shown inFig. 3Cto E. The C-terminal region of A/Texas/91 NS1 has the ability to interact with CPSF30, which is a component of the cellular pre-mRNA-processing machinery, and this interaction results in a lack of host mRNA polyadenylation and inducible host antiviral
FIG 5Characterization of the recombinant PR8-Tx NS1 WT and PR8-Tx NS1 CPSFb viruses in cell lines and human DCs. (A) Scheme of the recombinant viruses and the mutations introduced. (B) Growth kinetics of the viruses in MDCK and A549 cells. (C) Analysis of the expression of the NP, M, and NS1 viral genes in infected DCs by qRT-PCR. (D) Expression of viral HA, NP, M1, and NS1 protein levels and-actin in infected DCs at 12 h p.i. in Western blot analyses. One representative donor of at least three independent experiments with different donors is shown. Average results from replicates⫾SD are indicated.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:7.585.134.450.64.400.2]responses (35,36). This NS1 function might explain the reduced IFN-␣protein detection in the supernatants from PR8-Tx NS1 RNAb-infected DCs. Additionally, in contrast to the Tx NS1, the interaction of the NS1 from PR8 with CPSF30 is attenuated due to variations in two hydrophobic amino acids in the central region of the NS1 (Tx, 103F and 106M; PR8, 103S and 106I) in that virus (35). This results in a lack of CPSF30 inhibitory function in the NS1 of PR8, supporting the hypothesis of an important role of the CPSF30 binding domain in the inhibition of the production of type I IFN and DC activation.
To test if the posttranscriptional modification of IFN-␣/
RNA was altered in cells infected with the virus bearing the defec-tive dsRNA binding Tx NS1 (PR8-Tx NS1 RNAb), we analyzed the expression levels of IFN-␣ and IFN- RNAs by qRT-PCR, in which we reverse transcribed total RNA using random primers versus oligo(dT) primers that were specific for polyadenylated mRNA. Samples of 500 ng of RNA from infected DCs were sub-mitted to the reverse transcription reaction with random primers and 500 ng of RNA with oligo(dT) primers, and the expression levels of IFN-␣and IFN-were analyzed by qRT-PCR. As shown inFig. 4C, we observed that the induction of IFN RNA in DCs infected with PR8-Tx NS1 RNAb was higher than that in PR8-Tx
FIG 6Comparison of the cytokine expression profiles induced by PR8-Tx NS1 WT and PR8-Tx NS1 CPSFb in DCs. (A and D) qRT-PCR analyses of IFN-␣, IFN-, TNF-␣, and IL-6 in infected DCs. (B and E) IFN-␣, TNF-␣, and IL-6 protein detection in supernatants of infected cells by multiplex ELISA. (C) Analysis of IRF3-P and IRF3 levels by Western blotting and the IRF3-P/IRF3 ratios of the mean pixel intensities. One representative donor of at least three independent experiments with different donors is shown. Average results from replicates⫾SD are indicated. Statistically significant differences (Pⱕ0.05) between PR8-Tx NS1 CPSFb or PR8 dNS1 viruses and PR8-Tx NS1 WT were determined by the Studentttest and are represented by asterisks.
Influenza Virus NS1 Antagonist Functions in Human DCs
on November 7, 2019 by guest
http://jvi.asm.org/
[image:8.585.48.538.62.520.2]NS1 WT-infected DCs when random primers were used. Specifi-cally, the fold induction over the PR8-Tx NS1 WT-infected cells was 2.8 and 3 times higher for IFN-␣and IFN-, respectively, than for the oligo(dT)-processed samples, indicating that the poly-adenylation of the pre-mRNAs is not efficient in those cells, which might account for the reduced levels of IFN-␣protein in the PR8-Tx NS1 RNAb-infected cells in the presence of IRF3 phos-phorylation. In contrast, we did not observe significant increases in the expression of RNAs corresponding to other cytokines, such as TNF-␣and IL-6, when using either random or oligo(dT) prim-ers in PR8-Tx NS1 RNAb-infected cells over that in PR8-Tx NS1 WT-infected cells (Fig. 4C). It is intriguing that the dsRNA bind-ing function of the Tx NS1 recombinant virus is important for inhibiting the induction of type I IFN mRNA production but not TNF-␣and IL-6 mRNAs, while the entire NS1 protein is required for the inhibition of type I IFN induction and other cytokines, as shown inFig. 3and4and in reference22using a dNS1 mutant virus. The production of the proinflammatory cytokines TNF-␣ and IL-6, characteristic of DC activation, is driven by the activa-tion of the NF-〉 transcription factors (33, 37–39). Also, it is known that type I IFN induces NF-〉activation (reviewed in references31and40), so it is possible that the induction of TNF-␣ and IL-6 observed in dNS1 PR8-infected cells is mainly due to IFN secretion in those infected cells that in turn promotes activation of NF-〉. Thus, the lack of IFN-␣/protein production in PR8-Tx NS1 RNAb-infected cells might account for the lack of induction of TNF-␣and IL-6, since the feedback loop is absent.
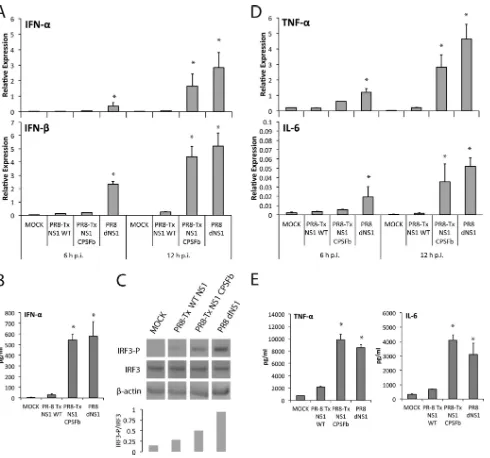
CPSF30 binding domain of the A/Texas/36/91 NS1 is impor-tant for inhibition of type I IFN production and activation of human DCs.The previous experiments pointed to a relevant con-tribution of the CPSF30 binding domain of Tx NS1 in the activa-tion of DCs and type I IFN producactiva-tion. We therefore proceeded to study the contribution of the Tx NS1 CPSF30 binding domain to the profile of DC activation induced by Tx NS1-expressing virus by generating a virus in a PR8 backbone bearing the Tx NS1 with mutations in the CPSF30 interaction site. Thus, amino acids 184 to 188 were changed from GLEWN to RFLRY, as depicted inFig. 5A(PR8-Tx NS1 CPSFb). This mutation is known to abrogate NS1 binding to CPSF30 without affecting the NEP protein se-quence (35,36). We first studied the replication kinetics of this virus in MDCK and A549 cell lines. As shown inFig. 5B, the PR8-Tx NS1 CPSFb mutant virus had replication profiles similar to that of the Tx NS1 WT-bearing virus. No significant differences in replication and expression levels of the viral proteins M, NP, and NS1 by qRT-PCR (Fig. 5C) or of HA, NP, NS1, and M1 by Western blotting (Fig. 5D) were observed when DCs were infected at an MOI of 0.5. In terms of type I IFN induction, we observed that the mutant virus PR8-Tx NS1 CPSFb induced higher levels of IFN-␣and IFN-mRNA than the PR8-Tx NS1 WT virus (32.4 and 17.1 at 12 h p.i., respectively) and levels similar to that of the PR8 dNS1-infected cells by qRT-PCR (Fig. 6A). These results were also confirmed by multiplex ELISA, suggesting efficient posttran-scriptional processing of IFN-␣/mRNAs in the PR8-Tx NS1 CPSFb-infected cells (Fig. 6B). Surprisingly, PR8-Tx NS1 CPSFb also induced higher levels of IRF3-P than PR8-Tx NS1 WT at 4 h p.i. (Fig. 6C), suggesting a previously undescribed role of the ef-fector domain of NS1 in IRF3 activation. As for the expression of proinflammatory cytokines in infected DCs, expression levels of TNF-␣and IL-6 measured by qRT-PCR and ELISA showed sim-ilar results (Fig. 6DandE), with higher levels of those cytokines
produced in PR8-Tx NS1 CPSFb- than in PR8-Tx NS1 WT-in-fected cells. Therefore, these data indicate that the CPSF30 bind-ing domain of NS1 is important for its innate immunity antago-nist role in primary human DCs, as amino acid residues in this domain are required for inhibiting the production of type I IFN and the expression of cytokines typical of DC activation, such as TNF-␣and IL-6.
Due to the involvement of the CPSF30 binding domain in mul-tiple cellular events, it is possible that the effect of the mutations introduced in positions 184 to 188 can affect functions other than CPSF30 binding, such as inhibition of IRF3 phosphorylation. Thus, the study of NS1 functions by mutagenesis should be per-formed carefully, as few amino acid changes might impact differ-ent NS1 functions.
The amino acids 103F and 106M, found in the A/Texas/36/91 NS1 isolate, are very conserved among human influenza strains (41,42), allowing efficient CPSF30 binding function. On the con-trary, NS1 from H5N1 avian influenza viruses, isolated from hu-mans before 1997, does not present these amino acids, showing low binding affinity to CPSF30in vitroand resulting in reduced viral replication in cell culture (43). Interestingly, another study from the same group indicated that influenza virus is able to over-come this defect with the help of other viral proteins (specifically NP and PA) which participate in the stabilization of the NS1-CPSF30 complex (44). In addition, H5N1 viruses isolated from humans after 1998 present the amino acids F103 and M106,
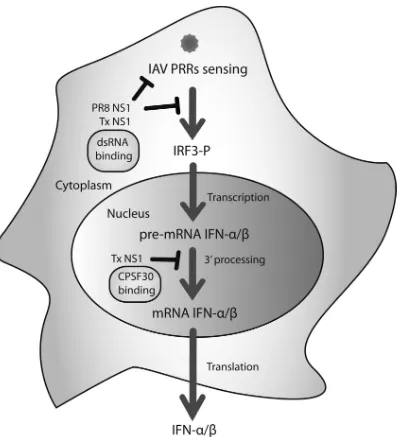
cor-FIG 7Schematic representation of the type I IFN inhibitory functions of influenza virus NS1 in human DCs. The dsRNA binding function of NS1 avoids viral sensing and activation of IRF3. This function is present in the NS1 of both PR8 and Tx. The CPSF30 binding function of NS1 inhibits 3= process-ing of IFN-␣/pre-mRNA, leading to the inhibition of protein synthesis. This function is present in the Tx NS1 but not in the PR8 NS1. Therefore, infection of DCs with a virus bearing Tx NS1 with the dsRNA binding domain altered results in IRF3 phosphorylation and IFN-␣/pre-mRNA synthesis. However, the CPSF30 binding domain of the NS1 of this virus inhibits the 3=processing of IFN-␣/pre-mRNA and, therefore, subsequent protein production. How-ever, altering the dsRNA binding domain of PR8 NS1 is sufficient to disrupt its type I IFN antagonistic role, since the PR8 NS1 does not possess a CPSF30
binding function.
on November 7, 2019 by guest
http://jvi.asm.org/
[image:9.585.317.524.64.285.2]recting the defective CPSF30 binding and showing increased vir-ulence (43). Altogether, these studies suggest that the NS1 CPSF30 binding function is an important feature for adaptation and viral fitness in humans. Interestingly, the new swine origin pandemic H1N1 virus encodes an NS1 protein deficient in CPSF30 binding (45).
In conclusion, this study provides a deeper knowledge of the NS1 inhibitory mechanisms of the innate immunity in human DCs. As depicted inFig. 7, the dsRNA binding domain of NS1 is important for the inhibition of type I IFN transcription as a con-sequence of the role of this function in avoiding viral sensing and activation of IRF3 but not of protein synthesis. However, the CPSF30 binding domain of NS1 is important for the inhibition of type I IFN protein production in influenza virus-infected DCs (Fig. 7). Also, we show that the CPSF30 binding ability of NS1 is responsible for counteracting the production of cytokines related with DC activation. The study of the NS1 functions in these im-portant immune cells is of great relevance in order to understand how IAV is able to counteract and evade innate and adaptive im-munity.
ACKNOWLEDGMENTS
We thank Juan Ayllón, Ricardo Rajsbaum, Randy Allen Albrecht, Alan Belicha-Villanueva, and all the Fernandez-Sesma lab members for re-agents and helpful suggestions and discussions. We also thank the Micros-copy Shared Facility at Mount Sinai School of Medicine for assistance.
This study is partly funded by NIH/NIAID grant 1R01AI073405 (to A.F.-S.), by NIH/NIAID contract CRIP, by the CEIRS Network Center for Research on Influenza Pathogenesis (supported by HHSN266200700010C [to A.G.-S. and A.F.-S.], by NIAID grants R01AI046954 and U19AI083025, and by the NIAID Mucosal Immunity Study Team (MIST) program under grant U01AI095611 (to A.G.-S.).
REFERENCES
1.Hale BG, Randall RE, Ortin J, Jackson D.2008. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol.89:2359 –2376.
2.Qian XY, Alonso-Caplen F, Krug RM.1994. Two functional domains of the influenza virus NS1 protein are required for regulation of nuclear export of mRNA. J. Virol.68:2433–2441.
3.Talon J, Horvath CM, Polley R, Basler CF, Muster T, Palese P, Garcia-Sastre A.2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol.74:7989 –7996.
4.Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, Garcia-Sastre A. 2000. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J. Virol.74:11566 –11573. 5.Min JY, Krug RM.2006. The primary function of RNA binding by the
influenza A virus NS1 protein in infected cells: inhibiting the 2=-5= oligo(A) synthetase/RNase L pathway. Proc. Natl. Acad. Sci. U. S. A.103: 7100 –7105.
6.Bergmann M, Garcia-Sastre A, Carnero E, Pehamberger H, Wolff K, Palese P, Muster T.2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol.74:6203– 6206.
7.Hatada E, Saito S, Fukuda R.1999. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J. Virol.73:2425–2433.
8.Lu Y, Wambach M, Katze MG, Krug RM.1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology214:222–228.
9.Tan SL, Katze MG.1998. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J. Interferon Cytokine Res.18:757–766. 10. Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan
M, Inoue S, Jung JU, Garcia-Sastre A.2009. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe5:439 – 449.
11. Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr, Garcia-Sastre A.2007. Inhibition of retinoic acid-inducible gene I-medi-ated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol.81:514 –524.
12. Qiu Y, Krug RM.1994. The influenza virus NS1 protein is a poly(A)-binding protein that inhibits nuclear export of mRNAs containing poly(A). J. Virol.68:2425–2432.
13. Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM.1998. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3=end formation of cellular pre-mRNAs. Mol. Cell1:991–1000. 14. Chen Z, Li Y, Krug RM.1999. Influenza A virus NS1 protein targets
poly(A)-binding protein II of the cellular 3=-end processing machinery. EMBO J.18:2273–2283.
15. Marazzi I, Ho JS, Kim J, Manicassamy B, Dewell S, Albrecht RA, Seibert CW, Schaefer U, Jeffrey KL, Prinjha RK, Lee K, Garcia-Sastre A, Roeder RG, Tarakhovsky A.2012. Suppression of the antiviral response by an influenza histone mimic. Nature483:428 – 433.
16. Aragon T, de la Luna S, Novoa I, Carrasco L, Ortin J, Nieto A.2000. Eukaryotic translation initiation factor 4GI is a cellular target for NS1 protein, a translational activator of influenza virus. Mol. Cell. Biol.20: 6259 – 6268.
17. Burgui I, Aragon T, Ortin J, Nieto A.2003. PABP1 and eIF4GI associate with influenza virus NS1 protein in viral mRNA translation initiation complexes. J. Gen. Virol.84:3263–3274.
18. Hale BG, Jackson D, Chen YH, Lamb RA, Randall RE.2006. Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc. Natl. Acad. Sci. U. S. A.103:14194 –14199. 19. Hale BG, Kerry PS, Jackson D, Precious BL, Gray A, Killip MJ, Randall
RE, Russell RJ.2010. Structural insights into phosphoinositide 3-kinase activation by the influenza A virus NS1 protein. Proc. Natl. Acad. Sci. U. S. A.107:1954 –1959.
20. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulen-dran B, Palucka K.2000. Immunobiology of dendritic cells. Annu. Rev. Immunol.18:767– 811.
21. Fernandez-Sesma A, Marukian S, Ebersole BJ, Kaminski D, Park MS, Yuen T, Sealfon SC, Garcia-Sastre A, Moran TM.2006. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol.80: 6295– 6304.
22. Haye K, Burmakina S, Moran T, Garcia-Sastre A, Fernandez-Sesma A. 2009. The NS1 protein of a human influenza virus inhibits type I inter-feron production and the induction of antiviral responses in primary hu-man dendritic and respiratory epithelial cells. J. Virol.83:6849 – 6862. 23. Phipps-Yonas H, Seto J, Sealfon SC, Moran TM, Fernandez-Sesma A.
2008. Interferon-beta pretreatment of conventional and plasmacytoid hu-man dendritic cells enhances their activation by influenza virus. PLoS Pathog.4:e1000193. doi:10.1371/journal.ppat.1000193.
24. Ramos I, Bernal-Rubio D, Durham N, Belicha-Villanueva A, Lowen AC, Steel J, Fernandez-Sesma A.2011. Effects of receptor binding spec-ificity of avian influenza virus on the human innate immune response. J. Virol.85:4421– 4431.
25. Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A.1999. Rescue of influenza A virus from recombinant DNA. J. Virol.73:9679 –9682.
26. Livak KJ, Schmittgen TD.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta CT[]) method. Methods25:402– 408.
27. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F.2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol.3:RESEARCH0034.
28. Park MS, Shaw ML, Munoz-Jordan J, Cros JF, Nakaya T, Bouvier N, Palese P, Garcia-Sastre A, Basler CF. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol.77: 1501–1511.
29. Solórzano A, Webby RJ, Lager KM, Janke BH, Garcia-Sastre A, Richt JA.2005. Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. J. Virol.79:7535– 7543.
30. Kerry PS, Ayllon J, Taylor MA, Hass C, Lewis A, Garcia-Sastre A, Randall RE, Hale BG, Russell RJ.2011. A transient homotypic interac-tion model for the influenza A virus NS1 protein effector domain. PLoS One6:e17946. doi:10.1371/journal.pone.0017946.
Influenza Virus NS1 Antagonist Functions in Human DCs
on November 7, 2019 by guest
http://jvi.asm.org/
31. Wang W, Riedel K, Lynch P, Chien CY, Montelione GT, Krug RM. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA.5:195–205.
32. Greenspan D, Palese P, Krystal M.1988. Two nuclear location signals in the influenza virus NS1 nonstructural protein. J. Virol.62:3020 –3026. 33. Libermann TA, Baltimore D.1990. Activation of interleukin-6 gene
expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 10:2327–2334.
34. Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M.1997. Bacterial lipopolysaccharide stimulates the pro-duction of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J. Immunol.158:2919 –2925.
35. Kochs G, Garcia-Sastre A, Martinez-Sobrido L.2007. Multiple anti-interferon actions of the influenza A virus NS1 protein. J. Virol.81:7011– 7021.
36. Noah DL, Twu KY, Krug RM.2003. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3=end processing of cellular pre-mRNAS. Virology307:386 –395.
37. Collart MA, Baeuerle P, Vassalli P.1990. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol. Cell. Biol.10:1498 –1506.
38. Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. 1990. Kappa B-type enhancers are involved in
lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J. Exp. Med.171:35– 47.
39. Shinoda K, Nakagawa K, Kosaka T, Tanaka N, Maeda T, Kono H, Mizuno R, Kikuchi E, Miyajima A, Umezawa K, Oya M.2010. Regula-tion of human dendritic cells by a novel specific nuclear factor-kappaB inhibitor, dehydroxymethylepoxyquinomicin. Hum. Immunol.71:763– 770.
40. Pfeffer LM.2011. The role of nuclear factor kappaB in the interferon response. J. Interferon Cytokine Res.31:553–559.
41. Das K, Ma LC, Xiao R, Radvansky B, Aramini J, Zhao L, Marklund J, Kuo RL, Twu KY, Arnold E, Krug RM, Montelione GT.2008. Structural basis for suppression of a host antiviral response by influenza A virus. Proc. Natl. Acad. Sci. U. S. A.105:13093–13098.
42. Macken C, Lu H, Goodman J, Boykin L.2001. Options for the control of influenza IV, p 103–106.InThe value of a database in surveillance and vaccine selection. Elsevier Science, Amsterdam, Netherlands.
43. Twu KY, Kuo RL, Marklund J, Krug RM.2007. The H5N1 influenza virus NS genes selected after 1998 enhance virus replication in mamma-lian cells. J. Virol.81:8112– 8121.
44. Kuo RL, Krug RM.2009. Influenza A virus polymerase is an integral component of the CPSF30-NS1A protein complex in infected cells. J. Vi-rol.83:1611–1616.
45. Hale BG, Steel J, Medina RA, Manicassamy B, Ye J, Hickman D, Hai R, Schmolke M, Lowen AC, Perez DR, Garcia-Sastre A.2010. Inefficient control of host gene expression by the 2009 pandemic H1N1 influenza A virus NS1 protein. J. Virol.84:6909 – 6922.