Randomized Comparison of Everolimus-Eluting and
Sirolimus-Eluting Stents in Patients Treated With
Percutaneous Coronary Intervention
The Scandinavian Organization for Randomized Trials With Clinical
Outcome IV (SORT OUT IV)
Lisette Okkels Jensen, MD, DMSci, PhD; Per Thayssen, MD, DMSci;
Henrik Steen Hansen, MD, DMSci; Evald Høj Christiansen, MD, PhD; Hans Henrik Tilsted, MD;
Lars Romer Krusell, MD; Anton Boel Villadsen, MD; Anders Junker, MD, PhD;
Knud Nørregaard Hansen, MD; Anne Kaltoft, MD, PhD; Michael Maeng, MD, PhD;
Knud Erik Pedersen, MD, DMSci; Steen Dalby Kristensen, MD, DMSci;
Hans Erik Bøtker, MD, DMSci, PhD; Jan Ravkilde, MD, DMSci; Richardo Sanchez, MD;
Jens Aarøe, MD; Morten Madsen, MSc; Henrik Toft Sørensen, MD, DMSci, PhD;
Leif Thuesen, MD, DMSci; Jens Flensted Lassen, MD, PhD; for the Scandinavian Organization for
Randomized Trials With Clinical Outcome IV (SORT OUT IV) Investigators
Background—Among drug-eluting stents released to date, the sirolimus-eluting stent has demonstrated the least amount of late lumen loss, but its efficacy and safety have not been compared head-to-head with the next-generation everolimus-eluting stent.
Methods and Results—The Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV) trial was a randomized multicenter, single-blind, all-comer, 2-arm, noninferiority trial comparing the everolimus-eluting stent with the sirolimus-eluting stent in patients with coronary artery disease. The primary end point was a composite of safety (cardiac death, myocardial infarction, definite stent thrombosis) and efficacy (target vessel revascularization) parameters. The noninferiority criterion was a risk difference of 0.015. Intention-to-treat analyses were done at 9- and 18-month follow-ups. A total of 1390 patients were assigned to receive the everolimus-eluting stent and 1384 patients to the sirolimus-eluting stent. At the 9-month follow-up, 68 patients (4.9%) treated with the everolimus-eluting stent compared with 72 patients (5.2%) treated with the sirolimus-eluting stent experienced the primary end point (hazard ratio, 0.94; 95% confidence interval, 0.67–1.31;Pfor noninferiority⫽0.01). At the 18-month follow-up, this differential remained: 99 patients (7.2%) treated with the everolimus-eluting stent versus 105 (7.6%) treated with the sirolimus-eluting stent (hazard ratio, 0.94; 95% confidence interval, 0.71–1.23). At the 9-month follow-up, the rate of definite stent thrombosis was higher in the sirolimus-eluting group (2 patients [0.1%] versus 9 patients [0.7%]; hazard ratio, 0.22; 95% confidence interval, 0.05–1.02). At the 18-month follow-up, this difference was sustained (3 patients [0.2%] versus 12 patients [0.9%]; hazard ratio, 0.25; 95% confidence interval, 0.07– 0.88).
Conclusion—The everolimus-eluting stent was found to be noninferior to the sirolimus-eluting stent.
Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: NCT00552877. (Circulation. 2012;125:1246-1255.)
Key Words:drug-eluting stent 䡲 revascularization 䡲 treatment outcome
Received August 23, 2011; accepted January 26, 2012.
From the Department of Cardiology, Odense University Hospital, Odense (L.O.J., P.T., H.S.H., A.J., K.N.H., K.E.P.); Department of Cardiology, Aarhus University Hospital, Skejby Hospital, Aarhus (E.H.C., L.R.K., A.K., M. Maeng, S.D.K., H.E.B., L.T., J.F.L.); Department of Cardiology, Aarhus University Hospital, Aalborg Hospital, Aalborg (H.H.T., A.B.V., J.R., J.A.); HeartCenter Varde, Varde (R.S.); and Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark (M. Madsen, H.T.S.).
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA. 111.063644/-/DC1.
Correspondence to Lisette Okkels Jensen, MD, DMSci, PhD, Department of Cardiology, Odense University Hospital, Sdr Blvd 29, 5000 Odense C, Denmark. E-mail okkels@dadlnet.dk
© 2012 American Heart Association, Inc.
Circulationis available at http://circ.ahajournals.org DOI: 10.1161/CIRCULATIONAHA.111.063644
I
n percutaneous coronary interventions, drug-eluting stent implantation is used increasingly for revascularization in patients with coronary artery disease. Compared with bare metal stents, first-generation drug-eluting stents, such as sirolimus- and paclitaxel-eluting stents, have shown im-proved results, reducing the need for repeat revascularization, as assessed in randomized trials.1–3Although drug-eluting stents are widely accepted as effec-tive and safe, debate continues on the safety of first-generation drug-eluting stents, given the potential for late stent thrombosis, especially after discontinuation of dual antiplatelet therapy.4,5Second-generation drug-eluting stents
were designed to improve efficacy, safety, and device per-formance. However, the first commercially available second-generation drug-eluting stent, the zotarolimus-eluting En-deavor stent, did not appear superior to the sirolimus-eluting stent in routine practice.6The next second-generation
drug-eluting stent, the everolimus-drug-eluting stent, proved superior to the paclitaxel-eluting stent, with a lower rate of stent throm-bosis, myocardial infarction, and target vessel revasculariza-tion7 and reduced angiographic late loss.8 In a recent
all-comer trial of second-generation drug-eluting stents, the zotarolimus-eluting stent proved noninferior to the everolimus-eluting stent despite a higher 1-year rate of definite stent thrombosis in the zotarolimus group.9
Clinical Perspective on p 1255
The Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV) trial aimed to compare the safety and efficacy of the first-generation sirolimus-eluting Cypher Select⫹ stent and the second-generation everolimus-eluting Xience V/Promus stent in a population-based setting using registry detection of clinically driven events.
Methods
Patients and Study DesignSORT OUT IV10 is a randomized, multicenter, single-blind, all-comer, 2-arm, noninferiority trial comparing the everolimus-eluting stent and the sirolimus-eluting stent in treating atherosclerotic coronary artery lesions. The study period was August 2007 to June 2009. Danish registry data11–13 were used to compare patients eligible for randomization who did not enroll with patients randomly allocated to treatment (Table 1), as required by the Consolidated Standards of Reporting Trials (CONSORT) statement.14
Eligible patients were at least 18 years of age and had chronic stable coronary artery disease or acute coronary syndromes and at least 1 coronary artery lesion with⬎50% diameter stenosis requiring treatment with a drug-eluting stent. If multiple lesions were treated, the allocated study stent had to be used in all lesions. No restrictions were placed on the number of treated lesions, number of treated vessels, or lesion length. Exclusion criteria were life expectancy of
⬍1 year; an allergy to aspirin, clopidogrel, sirolimus, or everolimus; participation in another randomized trial; or inability to provide written informed consent. The study complied with the Declaration of Helsinki and was approved by the local ethics committee. All patients provided written informed consent for trial participation. Randomization
Patients were enrolled by the investigators and randomly allocated to treatment groups after diagnostic coronary angiography and before percutaneous coronary intervention. Block randomization by center (permuted blocks of random sizes [2/4/6]) was used to assign
patients in a 1:1 ratio to receive the everolimus-eluting stent (Xience V, Abbott Vascular, or PROMUS, Abbott’s privately labeled Xience V Everolimus Eluting Coronary Stent System distributed by Boston Scientific Corp) or the sirolimus-eluting stent (Cypher Select⫹, Cordis, Johnson &Johnson). An independent organization computer generated the allocation sequence, stratified by sex and presence of diabetes mellitus. Patients were assigned to treatment through an automated telephone allocation service. Although operators were unblinded, all patients and individuals analyzing data were masked to treatment assignment.
Study Procedures
The everolimus-eluting stent was available in 6 diameters (2.25, 2.50, 2.75, 3.00, 3.5, and 4.00 mm) and 6 lengths (8, 12, 15, 18, 23, and 28 mm). The sirolimus-eluting stent was available in 5 diameters (2.25, 2.50, 2.75, 3.00, and 3.50 mm) and 6 lengths (8, 13, 18, 23, 28, and 33 mm). Stents were implanted according to standard tech-niques. Direct stenting without prior balloon dilation was allowed. Full lesion coverage was attempted by implanting ⬎1 stents. Drug-eluting stents not specified by the random allocation scheme and bare metal stents were prohibited unless the study stent could not be implanted. In such cases, other stents or balloon angioplasty alone was allowed. Before implantation, patients received at least 75 mg aspirin, a 600-mg loading dose of clopidogrel, and a dose of unfractionated heparin dose (5000 IU or 70 –100 IU/kg). Glycopro-Table 1. Baseline Demographic and Clinical Characteristics of Patients Who Were and Were Not Randomly Assigned to Treatments Groups Randomized (n⫽2774), n (%) Not Randomized (n⫽3952), n (%) P Age, mean (SD), y 64.1 (10.8) 63.5 (13.2) 0.045 Men 2098 (75.5) 2861 (72.4) 0.004 Diabetes mellitus 390/2774 (14.0) 438/2889 (15.2) 0.23 Current smoker 698/2342 (29.8) 949/2777 (34.2) ⬍0.001 Indication for percutaneous
coronary intervention ⬍0.001 ST-segment–elevation myocardial infarction 267 (9.6) 1334 (33.8) Non–ST-segment– elevation myocardial infarction or unstable angina 911 (32.8) 1149 (28.8) Stable angina 1530 (55.1) 1396 (35.3) Other 69 (2.5) 82 (2.1)
Target lesions per patient 0.006 1 1984 (71.4) 2883 (73.0)
2 593 (21.4) 835 (21.1) ⱖ3 200 (7.2) 234 (5.9)
Treated vessels per patient ⬍0.001 1 2247 (80.9) 3310 (83.8)
2 477 (17.2) 599 (15.2) 3 53 (1.9) 43 (1.1)
Comorbidity index score 0.018 0 1236 (44.5) 1755 (44.4)
1–2 1154 (41.6) 1553 (39.3) ⱖ3 387 (13.9) 644 (16.3)
All-cause mortality at 30 d 21 (0.8) 72 (1.8) ⬍0.001 Source: Western Denmark Heart Registry.
tein IIb/IIIa inhibitors were used at the operator’s discretion. Rec-ommended postprocedural dual antiplatelet regimens were 75 mg aspirin daily lifelong and 75 mg clopidogrel daily for 1 year. End Points
The primary end point was a combination of safety (cardiac death, myocardial infarction, definite stent thrombosis) and efficacy (clin-ically indicated target vessel revascularization) parameters within 9 months of stent implantation. Intention-to-treat analyses were con-ducted after 9 and 18 months of follow-up. Individual components of the primary end point comprised the secondary end points: cardiac death rate; myocardial infarction rate; definite stent thrombosis rate; rate of clinically indicated target vessel revascularization; rate of probable, possible, and overall stent thrombosis according to the Academic Research Consortium (ARC) definition15; symptom-driven target lesion revascularization; and device failure (defined as inability to implant the assigned study stent in⬎1 target lesions). Definitions
The study end points were defined as follows:
● Cardiac death: any death resulting from an evident cardiac cause, any death related to percutaneous coronary intervention, an unwit-nessed death, or death from unknown causes.
● Myocardial infarction: the universal definition used by the Euro-pean Society of Cardiology, American College of Cardiology, American Heart Association, and World Heart Federation.16 Bio-markers were not assessed at the time of the index percutaneous coronary intervention procedure.
● Stent thrombosis: definite, probable, or possible stent thrombosis according to the ARC15definition.
● Target vessel revascularization: any repeat percutaneous coronary intervention or surgical bypass of any segment within the entire major coronary vessel that was proximal or distal to a target lesion, including upstream and downstream branches, and the target lesion itself.
● Target lesion revascularization: repeat revascularization caused by a ⬎50% stenosis within the stent or within a 5-mm border proximal or distal to the stent. Target vessel and target lesion revascularization were clinically driven.
● Comorbidity: For all patients, we obtained data on all hospital diagnoses from the Danish National Registry of Patients covering all Danish hospitals from 1977 until the implantation date.11We then computed Charlson Comorbidity Index score, which covers 19 major disease categories, including diabetes mellitus, heart failure, cerebrovascular diseases, and cancer.17
Clinical Event Detection
Clinically driven event detection was used to avoid study-induced reinterventions. Data on mortality, hospital admission, coronary angiography, repeat percutaneous coronary intervention, and coro-nary bypass surgery were obtained for all randomly allocated patients from the following national Danish administrative and healthcare registries: the Civil Registration System; the Western Denmark Heart Registry12; the Danish National Registry of Pa-tients,11which maintains records on all hospitalizations in Denmark; and the Danish Registry of Causes of Death.13Independent event committee members who were blinded to treatment group assign-ment during the adjudication process reviewed all end points and source documents to adjudicate causes of death, reasons for hospi-talization, and diagnosis of myocardial infarction; they reviewed cine films to classify stent thrombosis and target vessel revascularization (with either percutaneous coronary intervention or coronary artery bypass grafting).
The Danish National Health Service provides universal tax-supported health care, guaranteeing residents free access to general practitioners and hospitals. The Danish Civil Registration System has kept electronic records on sex, birth date, residence, emigration date, and vital status changes since 196818with daily updates; the 10-digit civil registration number assigned at birth and used in all
registries allows accurate record linkage. The Civil Registration System provided vital status data for our study participants and minimized loss to follow-up. The National Registry of Causes of Deaths and the Danish National Registry of Patients provided information on causes of death and diagnoses assigned by the treating physician during hospitalizations (coded according to the International Classification of Diseases, 10th revision).11
Statistical Analysis
The trial was powered for assessing the noninferiority of the everolimus-eluting stent to the sirolimus-eluting stent with respect to the primary end point at 9 months. An event rate of 0.0688was assumed in the everolimus-eluting stent group and 0.07819 in the sirolimus-eluting stent group, with an expected difference in event rates between the 2 groups of⫺0.010 at 9 months. With a sample size of 1339 patients in each treatment arm, a 2-group large-sample normal approximation test of proportions with a 1-sided 0.050 significance level will have 80% power to detect noninferiority with a predetermined noninferiority margin of 0.015. The sample size of 1339 assumes a 0% lost-to-follow-up rate given the use of the Civil Registration System. A Farrington-Manning20test was used to test for noninferiority. Distributions of continuous variables were com-pared between study groups by use of the 2-samplettest (or Cochran test for cases of unequal variance) or the Mann-Whitney U test, depending on whether the data followed a normal distribution. Distributions of categorical variables were compared by use of the2
test. In analyses of every end point, follow-up continued until the date of an end-point event, death, or emigration or 18 months after stent implantation, whichever came first. Survival curves were constructed on the basis of time to events, accounting for the competing risk of death. Hazard ratios were computed with Cox proportional hazards regression analysis. Patients treated with the sirolimus-eluting stent were used as the reference group for overall and subgroup analyses. Hazard ratios were calculated for major adverse cardiac events at the 18-month follow-up for prespecified patient subgroups (based on baseline demographic and clinical characteristics). The intention-to-treat principle was used in all analyses. Except for the inferiority testing of the primary end point, a 2-sided value of P⬍0.05 was considered to indicate statistical significance. Analyses were conducted with SAS software (version 9.2). This trial is registered at http://www.clinicaltrials.gov (number NCT00552877).
Results
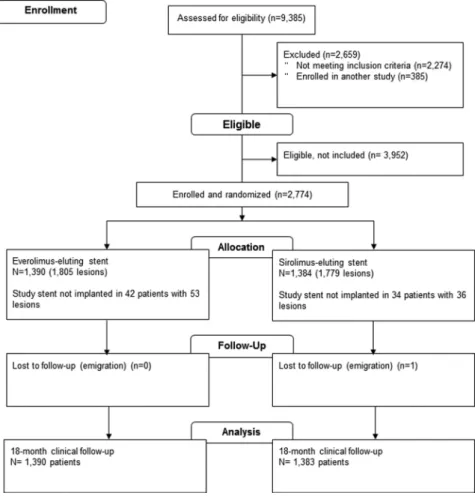
Patients and EnrollmentA total of 9385 patients were screened, and 2774 patients with 3584 lesions were randomly assigned to receive either the everolimus-eluting stent (1390 patients with 1805 lesions) or the sirolimus-eluting stent (1384 patients with 1779 le-sions). One patient was lost to follow-up on day 187 because of emigration (this person was considered a success [non-event] for the noninferiority analysis of the primary end point). The flow diagram of the trial is provided in Figure 1. Baseline characteristics were recorded for all patients eligible for randomization regardless of subsequent trial participation (Table 1). Eligible nonrandomized patients were older, were more often hospitalized with ST-segment– elevation myocar-dial infarction, and had higher 30-day mortality than random-ized patients.
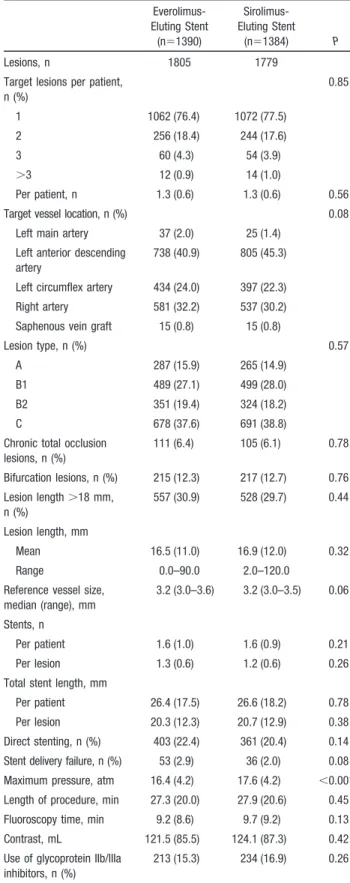
Baseline demographic and clinical characteristics were well balanced between the 2 study groups (Table 2). Patients in the sirolimus-eluting stent group had more left anterior descending coronary artery lesions. The maximum stent implantation pressure was higher in the sirolimus-eluting stent group (Table 3). A high proportion of patients in both groups had acute coronary syndromes, multivessel disease,
and complex lesions (Table 4). A total of 194 patients (14.0%) treated with everolimus-eluting stents had diabetes mellitus compared with 196 patients (14.2%) treated with sirolimus-eluting stents.
Clinical Outcomes
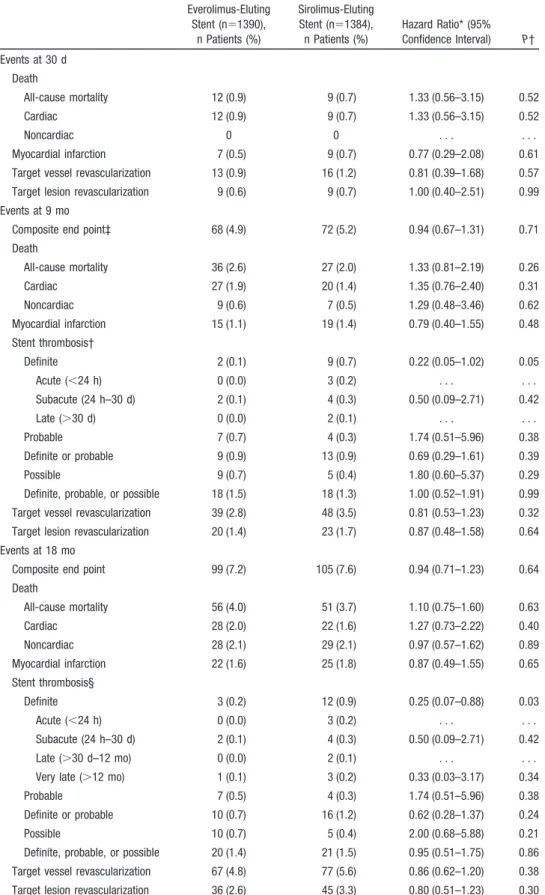
The composite primary end point occurred in 68 patients (4.9%) in the everolimus-eluting stent group and in 72 patients (5.2%) in the sirolimus-eluting stent group (Figure 2). Noninferiority of the everolimus-eluting stent was estab-lished, with a 9-month absolute risk difference of⫺0.3% and the upper limit of the 1-sided 95% confidence interval at 1.1% (1-sided P for noninferiority⫽0.02). Rates of death, cardiac death, myocardial infarction, clinically driven target vessel revascularization, and clinically driven target lesion revascularization did not differ significantly between the 2 stent groups (Table 4). The result was sustained for the composite end point at 18 months, which occurred in 99 patients (7.2%) in the everolimus-eluting stent group and in 105 patients (7.6%) in the sirolimus-eluting stent group (Table 4 and Figure 2). Definite stent thrombosis occurred within 9 months in 2 patients (0.1%) in the everolimus-eluting stent group and in 9 patients (0.7%) in the sirolimus-eluting stent group (P⫽0.053). At the 18-month follow-up, this difference was sustained (3 patients [0.2%] versus 12 patients [0.9%];P⫽0.03; Figure 2 and Table 4). (Because of small event rates, we further tested this difference with a Fisher exact test [P⫽0.021].) At the 18-month follow-up, definite or probable stent thrombosis did not differ between
the 2 groups; it occurred in 10 patients (0.7%) in the everolimus-eluting stent group and in 16 patients (1.2%) in the sirolimus-eluting stent group (P⫽0.24; Table 4). Probable stent thrombosis was caused by 7 unexplained deaths within the first 30 days in the everolimus-eluting stent group compared with 4 unexplained deaths within the first 30 days in the sirolimus-eluting stent group (Table 4). Findings for the primary end point were consistent across prespecified strati-fied analyses (Figure 3). The primary end point did not differ significantly between the 2 stent groups among patients with and without diabetes mellitus. Among diabetic patients, rates of major cardiac adverse events did not differ significantly between the everolimus-eluting and sirolimus-eluting stent groups at 18 months (10.3% and 15.8%, respectively; hazard ratio, 0.63; 95% confidence interval, 0.36 –1.11).
Discussion
Our trial provides the first head-to-head comparison of the everolimus-eluting stent and the sirolimus-eluting stent (con-sidered the most validated and efficient first-generation drug-eluting stent). We documented noninferiority of the everolimus-eluting stent overall, and across a variety of patient and lesion subgroups, the 2 treatments yielded similar composite endpoint results, including the presence of diabetes mellitus, acute coronary syndrome, complex lesions, and multivessel disease.
Rates of cardiac mortality, myocardial infarction, and target vessel revascularization did not differ significantly
between the 2 groups, although definite stent thrombosis was lower in the everolimus-eluting stent group.
Increased risk of late and very late stent thrombosis associated with first-generation drug-eluting stents led to recommendations for large-scale randomized clinical end-point trials encompassing a variety of patient categories and types of coronary lesions to allow a head-to-head comparison of drug-eluting stents with different stent platforms, poly-mers, and antiproliferative drugs. Extensive comparisons of sirolimus-eluting and paclitaxel-eluting stents demonstrated similar safety and probably higher efficacy for the sirolimus-eluting stent.19,21–23 A number of second-generation
drug-eluting stents have been developed, aiming to achieve reste-nosis rates similar to those of sirolimus-eluting and paclitaxel-eluting stents but with a better safety profile. Although the zotarolimus-eluting Endeavor stent did not reach the efficacy level of the sirolimus-eluting stent and showed no indication of improved safety,6 the
everolimus-eluting stent seemed promising in terms of both safety and efficacy. The Second-Generation Everolimus-Eluting and Paclitaxel-Eluting Stents in Real-Life Practice (COMPARE) trial7 and the Everolimus-Eluting Versus Paclitaxel-Eluting
Stents in Coronary Artery Disease (SPIRIT IV) trial24
com-paring everolimus-eluting and paclitaxel-eluting stents showed a favorable safety and efficacy profile for the everolimus-eluting stent. In the SPIRIT V trial,24 the
everolimus-eluting stent demonstrated lower rates of target
vessel failure; in the COMPARE trial,7 it demonstrated a
reduced rate of combined all-cause mortality, nonfatal myo-cardial infarction, target vessel revascularization, and early definite stent thrombosis compared with paclitaxel-eluting
Table 2. Baseline Characteristics of the Study Population Everolimus-Eluting Stent (n⫽1390), n (%) Sirolimus-Eluting Stent (n⫽1384), n (%) P Age, mean (SD), y 64.2 (10.9) 64.0 (10.8) 0.68 Men 1055 (75.9) 1041 (75.2) 0.68 Diabetes mellitus 194/1390 (14.0) 196/1384 (14.2) 0.88 Arterial hypertension 689/1215 (56.7) 649/1207 (53.8) 0.15 Hypercholesterolemia 866/1218 (71.1) 859/1208 (71.1) 1.00 Current smoker 344/1178 (29.2) 353/1162 (30.4) 0.53 Body mass index, mean
(SD), kg/m2 27.5 (4.7) 27.4 (4.4) 0.43 Previous myocardial infarction 276/1223 (22.6) 259/1214 (21.3) 0.46 Previous percutaneous coronary intervention 264/1227 (21.5) 250/1214 (20.6) 0.58 Previous coronary artery
bypass grafting
118/1227 (9.6) 97/1214 (8.0) 0.16 Indication for percutaneous
coronary intervention 0.46 ST-segment–elevation myocardial infarction 122 (8.8) 145 (10.5) Non–ST-segment– elevation myocardial infarction or unstable angina 458 (32.9) 453 (32.7) Stable angina 773 (55.6) 754 (54.4) Other 37 (2.7) 32 (2.3)
Table 3. Baseline Lesion and Procedure Characteristics Everolimus-Eluting Stent (n⫽1390) Sirolimus-Eluting Stent (n⫽1384) P Lesions, n 1805 1779 Target lesions per patient,
n (%) 0.85 1 1062 (76.4) 1072 (77.5) 2 256 (18.4) 244 (17.6) 3 60 (4.3) 54 (3.9) ⬎3 12 (0.9) 14 (1.0) Per patient, n 1.3 (0.6) 1.3 (0.6) 0.56 Target vessel location, n (%) 0.08
Left main artery 37 (2.0) 25 (1.4) Left anterior descending
artery
738 (40.9) 805 (45.3) Left circumflex artery 434 (24.0) 397 (22.3) Right artery 581 (32.2) 537 (30.2) Saphenous vein graft 15 (0.8) 15 (0.8)
Lesion type, n (%) 0.57 A 287 (15.9) 265 (14.9)
B1 489 (27.1) 499 (28.0) B2 351 (19.4) 324 (18.2) C 678 (37.6) 691 (38.8) Chronic total occlusion
lesions, n (%) 111 (6.4) 105 (6.1) 0.78 Bifurcation lesions, n (%) 215 (12.3) 217 (12.7) 0.76 Lesion length⬎18 mm, n (%) 557 (30.9) 528 (29.7) 0.44 Lesion length, mm Mean 16.5 (11.0) 16.9 (12.0) 0.32 Range 0.0–90.0 2.0–120.0
Reference vessel size, median (range), mm
3.2 (3.0–3.6) 3.2 (3.0–3.5) 0.06 Stents, n
Per patient 1.6 (1.0) 1.6 (0.9) 0.21 Per lesion 1.3 (0.6) 1.2 (0.6) 0.26 Total stent length, mm
Per patient 26.4 (17.5) 26.6 (18.2) 0.78 Per lesion 20.3 (12.3) 20.7 (12.9) 0.38 Direct stenting, n (%) 403 (22.4) 361 (20.4) 0.14 Stent delivery failure, n (%) 53 (2.9) 36 (2.0) 0.08 Maximum pressure, atm 16.4 (4.2) 17.6 (4.2) ⬍0.001 Length of procedure, min 27.3 (20.0) 27.9 (20.6) 0.45 Fluoroscopy time, min 9.2 (8.6) 9.7 (9.2) 0.13 Contrast, mL 121.5 (85.5) 124.1 (87.3) 0.42 Use of glycoprotein IIb/IIIa
inhibitors, n (%)
213 (15.3) 234 (16.9) 0.26 Data are mean (SD), number of patients (%), or median (interquartile range) as appropriate.
Table 4. Clinical Outcomes Everolimus-Eluting Stent (n⫽1390), n Patients (%) Sirolimus-Eluting Stent (n⫽1384), n Patients (%) Hazard Ratio* (95% Confidence Interval) P† Events at 30 d Death All-cause mortality 12 (0.9) 9 (0.7) 1.33 (0.56–3.15) 0.52 Cardiac 12 (0.9) 9 (0.7) 1.33 (0.56–3.15) 0.52 Noncardiac 0 0 . . . . Myocardial infarction 7 (0.5) 9 (0.7) 0.77 (0.29–2.08) 0.61 Target vessel revascularization 13 (0.9) 16 (1.2) 0.81 (0.39–1.68) 0.57 Target lesion revascularization 9 (0.6) 9 (0.7) 1.00 (0.40–2.51) 0.99 Events at 9 mo
Composite end point‡ 68 (4.9) 72 (5.2) 0.94 (0.67–1.31) 0.71 Death All-cause mortality 36 (2.6) 27 (2.0) 1.33 (0.81–2.19) 0.26 Cardiac 27 (1.9) 20 (1.4) 1.35 (0.76–2.40) 0.31 Noncardiac 9 (0.6) 7 (0.5) 1.29 (0.48–3.46) 0.62 Myocardial infarction 15 (1.1) 19 (1.4) 0.79 (0.40–1.55) 0.48 Stent thrombosis† Definite 2 (0.1) 9 (0.7) 0.22 (0.05–1.02) 0.05 Acute (⬍24 h) 0 (0.0) 3 (0.2) . . . . Subacute (24 h–30 d) 2 (0.1) 4 (0.3) 0.50 (0.09–2.71) 0.42 Late (⬎30 d) 0 (0.0) 2 (0.1) . . . . Probable 7 (0.7) 4 (0.3) 1.74 (0.51–5.96) 0.38 Definite or probable 9 (0.9) 13 (0.9) 0.69 (0.29–1.61) 0.39 Possible 9 (0.7) 5 (0.4) 1.80 (0.60–5.37) 0.29 Definite, probable, or possible 18 (1.5) 18 (1.3) 1.00 (0.52–1.91) 0.99 Target vessel revascularization 39 (2.8) 48 (3.5) 0.81 (0.53–1.23) 0.32 Target lesion revascularization 20 (1.4) 23 (1.7) 0.87 (0.48–1.58) 0.64 Events at 18 mo
Composite end point 99 (7.2) 105 (7.6) 0.94 (0.71–1.23) 0.64 Death All-cause mortality 56 (4.0) 51 (3.7) 1.10 (0.75–1.60) 0.63 Cardiac 28 (2.0) 22 (1.6) 1.27 (0.73–2.22) 0.40 Noncardiac 28 (2.1) 29 (2.1) 0.97 (0.57–1.62) 0.89 Myocardial infarction 22 (1.6) 25 (1.8) 0.87 (0.49–1.55) 0.65 Stent thrombosis§ Definite 3 (0.2) 12 (0.9) 0.25 (0.07–0.88) 0.03 Acute (⬍24 h) 0 (0.0) 3 (0.2) . . . . Subacute (24 h–30 d) 2 (0.1) 4 (0.3) 0.50 (0.09–2.71) 0.42 Late (⬎30 d–12 mo) 0 (0.0) 2 (0.1) . . . . Very late (⬎12 mo) 1 (0.1) 3 (0.2) 0.33 (0.03–3.17) 0.34 Probable 7 (0.5) 4 (0.3) 1.74 (0.51–5.96) 0.38 Definite or probable 10 (0.7) 16 (1.2) 0.62 (0.28–1.37) 0.24 Possible 10 (0.7) 5 (0.4) 2.00 (0.68–5.88) 0.21 Definite, probable, or possible 20 (1.4) 21 (1.5) 0.95 (0.51–1.75) 0.86 Target vessel revascularization 67 (4.8) 77 (5.6) 0.86 (0.62–1.20) 0.38 Target lesion revascularization 36 (2.6) 45 (3.3) 0.80 (0.51–1.23) 0.30 *From a Cox regression model.
†Two-sided from a Cox regression model.
‡Primary end point. Composite of major adverse cardiac events: cardiac death, myocardial infarction, definite stent thrombosis, and clinically driven target vessel revascularization.
stents. A comparison showed similar rates of target lesion failure in the zotarolimus-eluting Endeavor Resolute stent and the everolimus-eluting stent but a higher rate of definite stent thrombosis in the Resolute stent within the first 12
months.9 These results were sustained after 24 months;
however, the numbers of very late definite stent thromboses were equal in the 2 groups.25A positive safety profile for the
everolimus-eluting stent also emerged in long-term
registry-Figure 2.Time-to-event curves for major adverse cardiac events. Major adverse cardiac events are a composite of cardiac death, myo-cardial infarction, definite stent thrombosis, and target vessel revascularization.A, Major adverse cardiac events.B, Cardiac death.C, Myocardial infarction.D, Target vessel revascularization.E, Definite stent thrombosis.F, Definite or probable stent thrombosis.
Figure 3.Prespecified subgroup analysis for the primary end point at the 18-month follow-up. Data are presented by number of patients. Major adverse cardiac events are a composite of cardiac death, myocardial infarction, definite stent thrombosis, and target vessel revascularization. CI indicates confidence interval; LAD, left anterior descending artery; PCI, percutaneous coronary intervention; and STEMI, ST-segment– elevation myocardial infarction.
based follow-up studies and in the SPIRIT I through III studies.8,26 –28
Surprisingly, our study showed a significant association with less definite stent thrombosis in patients treated with the everolimus-eluting stent. This finding also was observed in the Comparison of Zotarolimus-Eluting and Everolimus-Eluting Coronary Stents (RESOLUTE) trial9 in which the
everolimus-eluting stent was associated with significantly less definite stent thrombosis than the zotarolimus-eluting Endeavor Resolute stent. The low rates of definite stent thrombosis observed in the present trial also were comparable to those in the everolimus-eluting groups in 3 large random-ized trials: COMPARE,7RESOLUTE,9and SPIRIT IV,24in
which the rates after 12 months were 0.4%, 0.3%, and 0.3%, respectively, according to the ARC definition.15The
favor-able stent thrombosis rate for the everolimus-eluting stent awaits confirmation in longer-term follow-up randomized studies. The definite stent thrombosis results were a second-ary end point that should be interpreted with caution and need replication to confirm that they are not spurious. A registry cohort study that compared the long-term performance of everolimus-eluting and sirolimus-eluting stents using propen-sity score matching found that definite stent thrombosis was less frequent among patients treated with everolimus-eluting stents.29 For the sirolimus-eluting stent group, the definite
stent thrombosis rate observed in our trial was comparable to that for the sirolimus-eluting stent in other all-comer studies.6,19
Definite stent thrombosis is documented most often through catheterization because autopsies are rare. Although the definite stent thrombosis definition maximizes specificity, it may be insufficiently sensitive to capture completely this relatively rare event. Sensitivity can be increased by includ-ing cases of probable and possible stent thrombosis in the analysis. In accordance with the ARC recommendation,15we
combined adjudicated definite and probable stent thrombosis events to obtain the best safety characterization of the 2 stents under investigation. We found that the combined definite or probable stent thrombosis rates in the 2 stent groups were equal.
Target vessel revascularization and target lesion revascu-larization were low in both groups but comparable to previ-ous studies without angiographic follow-up.6,7,19We found a
slightly lower reintervention rate in our everolimus-eluting stent group compared with the RESOLUTE trial,25 most
likely because of the 18-month follow-up interval in our study and the 24-month follow-up interval in the RESOLUTE trial. We also found a slightly lower reintervention rate in our sirolimus group compared with that in the Biolimus-Eluting Stent With Biodegradable Polymer Versus Sirolimus-Eluting Stent With Durable Polymer for Coronary Revascularization (LEADERS) trial.30 The Intracoronary Stenting and
Angio-graphic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) trial,31 which compared the
everolimus-eluting stent and the sirolimus-everolimus-eluting stent, found no differ-ence in performance efficacy between the 2 stent groups within 12 months. That trial included a scheduled angiogra-phy after 6 to 8 months, resulting in higher target vessel revascularization rates compared with our study, which, like
the earlier SORT OUT studies, relied on detection of clini-cally driven events from medical registries to avoid study-induced reinterventions.32
The SORT OUT II,19 III,6 and IV studies relied on
registry-based event detection without study-related angio-graphic or clinical follow-up. Patient care complied with normal clinical practice, ie, follow-up during a hospital outpatient visit after 1 to 3 months. We believe that this approach to event detection, combined with a randomized all-comer trial design, allowed us to assess the efficacy of different percutaneous coronary interventions in a context reflecting everyday clinical practice during the study period. Our findings showed fewer events, particularly fewer myo-cardial infarctions, than reported in other randomized tri-als.7,9,30This difference can be explained partly by
procedure-related myocardial infarction, which was not part of the primary end point in the SORT OUT IV trial. In the LEADERS trial,30 the myocardial infarction rate increased
0.5% and 0.6%, respectively, from 30 days (biolimus-eluting stent, 4.9%; sirolimus-eluting stent, 4.1%) to 9 months after implantation (biolimus-eluting stent, 5.7%; sirolimus-eluting stent, 4.6%), indicating that the majority of myocardial infarctions were early and related predominantly to stent implantation. It is important to note that our follow-up period of 18 months may be too short to assess the risk of very late stent thrombosis.
Like most stent trials, the SORT OUT IV trial was designed as a single-blind study, and we believe that the lack of double-blindness would not influence the results because all end points were objective and determined by event committee members who were blinded to treatment group assignment during the adjudication process. There was a lower rate of major adverse cardiac events in both treatment groups than assumed in the power calculation. At the time the study was designed, most randomized trials had on-label indications for enrollment, and the true rate of major adverse cardiac events was not available in the literature. In particu-lar, there were no data on the clinically driven event rate. The clinical outcomes after implantation of drug-eluting stents have improved in recent years. Therefore, we expect that the event rate in our study is representative of the real event rate among this patient population.
Conclusion
The everolimus-eluting stent was found to be noninferior to the sirolimus-eluting stent for patients treated with percuta-neous coronary intervention.
Acknowledgments
A complete list of the SORT OUT IV Study Group member can be found in the Appendix in the online-only Data Supplement. Contrib-utors: The Steering Committee formulated the study design, which all authors subsequently accepted. Drs Madsen and Sørensen were responsible for data management and for the design and implemen-tation of the statistical analysis. All other authors enrolled patients and contributed to data collection. Drs Okkels Jensen, Thayssen, Lassen, and Thuesen contributed to the design of the statistical analysis and the interpretation of results. Drs Okkels Jensen, Thayssen, Thuesen, and Lassen drafted the report, which was subsequently reviewed by all authors. All authors have seen the final submitted report and agree with its contents.
Sources of Funding
Equal unrestricted grants were received from Abbott Vascular, Boston Scientific, and Cordis, Johnson & Johnson. These companies did not have a role in study design, data collection, data analysis, or interpretation of results. They also did not have access to the clinical trial database or an opportunity to review the manuscript.
Disclosures
Dr Okkels Jensen has received honoraria from Abbott Vascular and Cordis (CEC member). Drs Thayssen, Villadsen, Aarøe, and San-chez have received unrestricted grants from Abbott Vascular and Cordis to their institutions. Dr Christiansen has received honoraria and travel grants from Abbott Vascular and Cordis. Dr Tilsted has received travel grants from Abbott Vascular and Cordis to his institution and is married to a Cordis employee. Dr Kaltoft has received unrestricted grants from Abbott Vascular, Boston Scientific, and Cordis to her institution and honoraria from Cordis. Dr Maeng has received travel grants from Cordis, Medtronic, and Boston Scientific. Dr Kristensen has received honoraria from Astra Zeneca, Eli Lilly, Daichii Sankyo, and The Medicine Company. Dr Ravkilde has received unrestricted grants from Abbott Vascular and Cordis to his institution and honoraria from Abbott Vascular. Dr Thuesen has received unrestricted grants from Abbott Vascular, Boston Scientific, and Cordis to his institution and honoraria from Abbott Vascular, Cordis, and Boston Scientific. Dr Lassen has received unrestricted grants from Abbott Vascular, Boston Scientific, and Cordis to his institution and speaking honoraria from Abbott Vascular, Cordis, Medtronic, Eli Lilly, Boston Scientific, and AstraZeneca. The other authors report no conflicts.
References
1. Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban HE, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization.N Engl J Med. 2002;346:1773–1780. 2. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery.N Engl J Med. 2003; 349:1315–1323.
3. Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease.N Engl J Med. 2004;350:221–231.
4. Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667– 678.
5. Jensen LO, Tilsted HH, Thayssen P, Kaltoft A, Maeng M, Lassen JF, Hansen KN, Madsen M, Ravkilde J, Johnsen SP, Sorensen HT, Thuesen L. Paclitaxel and sirolimus eluting stents versus bare metal stents: long-term risk of stent thrombosis and other outcomes. From the Western Denmark Heart Registry.EuroIntervention. 2010;5:898 –905. 6. Rasmussen K, Maeng M, Kaltoft A, Thayssen P, Kelbaek H, Tilsted HH,
Abildgaard U, Christiansen EH, Engstrom T, Krusell LR, Ravkilde J, Hansen PR, Hansen KN, Abildstrom SZ, Aaroe J, Jensen JS, Kristensen SD, Botker HE, Madsen M, Johnsen SP, Jensen LO, Sorensen HT, Thuesen L, Lassen JF. Efficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trial.Lancet. 2010;375:1090 –1099. 7. Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, Smits PC. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–209.
8. Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, Farhat N, Mahaffey KW, Cutlip DE, Fitzgerald PJ, Sood P, Su X, Lansky AJ. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial.JAMA. 2008;299:1903–1913.
9. Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbaek H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, Dimario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents.N Engl J Med. 2010;363:136 –146.
10. Jensen LO, Thayssen P, Tilsted HH, Ravkilde J, Junker A, Hansen HS, Hansen KN, Pedersen KE, Sorensen HT, Thuesen L, Lassen JF. rationale and design of a randomized clinical comparison of everolimus-eluting (Xience V/Promus) and sirolimus-eluting (Cypher Select⫹) coronary stents in unse-lected patients with coronary heart disease.Cardiology. 2010;116:73–78. 11. Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The
Danish National Hospital Register: a valuable source of data for modern health sciences.Dan Med Bull. 1999;46:263–268.
12. Jensen LO, Maeng M, Kaltoft A, Thayssen P, Hansen HH, Bottcher M, Lassen JF, Krussel LR, Rasmussen K, Hansen KN, Pedersen L, Johnsen SP, Soerensen HT, Thuesen L. Stent thrombosis, myocardial infarction, and death after drug-eluting and bare-metal stent coronary interventions. J Am Coll Cardiol. 2007;50:463– 470.
13. Juel K, Helweg-Larsen K. The Danish registers of causes of death.Dan Med Bull. 1999;46:354 –357.
14. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials.J Clin Epidemiol. 2010;63:e1– e37.
15. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions.Circulation. 2007;115:2344 –2351.
16. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction.Eur Heart J. 2007;28:2525–2538.
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis. 1987;40:373–383.
18. Frank L. Epidemiology; when an entire country is a cohort.Science. 2000;287:2398 –2399.
19. Galloe AM, Thuesen L, Kelbaek H, Thayssen P, Rasmussen K, Hansen PR, Bligaard N, Saunamaki K, Junker A, Aaroe J, Abildgaard U, Ravkilde J, Engstrom T, Jensen JS, Andersen HR, Botker HE, Galatius S, Kristensen SD, Madsen JK, Krusell LR, Abildstrom SZ, Stephansen GB, Lassen JF. Comparison of paclitaxel- and sirolimus-eluting stents in everyday clinical practice: the SORT OUT II randomized trial.JAMA. 2008;299:409 – 416.
20. Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk dif-ference or non-unity relative risk.Stat Med. 1990;9:1447–1454. 21. Morice MC, Colombo A, Meier B, Serruys P, Tamburino C, Guagliumi
G, Sousa E, Stoll HP. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA. 2006;295:895–904.
22. Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabate M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Juni P. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis.Lancet. 2007;370:937–948.
23. Schomig A, Dibra A, Windecker S, Mehilli J, Suarez de LJ, Kaiser C, Park SJ, Goy JJ, Lee JH, Di LE, Wu J, Juni P, Pfisterer ME, Meier B, Kastrati A. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease.J Am Coll Cardiol. 2007;50:1373–1380.
24. Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, Lansky AJ, Cutlip DE, Kereiakes DJ. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease.N Engl J Med. 2010;362:1663–1674. 25. Silber S, Windecker S, Vranckx P, Serruys PW. Unrestricted randomised
use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-patient-related outcomes from the RESOLUTE All Comers trial.Lancet. 2011;377:1241–1247.
26. Serruys PW, Ong AT, Piek JJ, Neumann FJ, van der Giessen WJ, Wiemer M, Zeiher A, Grube E, Haase J, Thuesen L, Hamm C, Otto-Terlouw PC. A randomized comparison of a durable polymer everolimus-eluting stent with a bare metal coronary stent: the SPIRIT first trial.EuroIntervention. 2005;1:58 – 65.
27. Serruys PW, Ruygrok P, Neuzner J, Piek JJ, Seth A, Schofer JJ, Richardt G, Wiemer M, Carrie D, Thuesen L, Boone E, Miquel-Herbert K, Daemen J. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent: the SPIRIT II trial. EuroIntervention. 2006;2:286 –294.
28. Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, Farhat N, Caputo R, Xenopoulos N, Applegate R, Gordon P, White RM, Sudhir K, Cutlip DE, Petersen JL. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With de novo Native Coronary Artery Lesions (SPIRIT) III trial.Circulation. 2009; 119:680 – 686.
29. Raber L, Juni P, Nuesch E, Kalesan B, Wenaweser P, Moschovitis A, Khattab AA, Bahlo M, Togni M, Cook S, Vogel R, Seiler C, Meier B, Windecker S. Long-term comparison of everolimus-eluting and sirolimus-eluting stents for coronary revascularization. J Am Coll Cardiol. 2011;57:2143–2151.
30. Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, Lenk K, Ischinger T, Klauss V, Eberli F, Corti R, Wijns W, Morice MC, Di MC, Davies S, van Geuns RJ, Eerdmans P, Van Es GA, Meier B, Juni P. Biolimus-Eluting Stent With Biodegradable Polymer Versus Sirolimus-Eluting Stent With Durable Polymer for Coronary Revascu-larisation (LEADERS): a randomised non-inferiority trial.Lancet. 2008; 372:1163–1173.
31. Byrne RA, Kastrati A, Kufner S, Massberg S, Birkmeier KA, Laugwitz KL, Schulz S, Pache J, Fusaro M, Seyfarth M, Schomig A, Mehilli J. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J. 2009;30:2441–2449.
32. Ruygrok PN, Melkert R, Morel MA, Ormiston JA, Bar FW, Fernandez-Aviles F, Suryapranata H, Dawkins KD, Hanet C, Serruys PW. Does angiography six months after coronary intervention influence man-agement and outcome? Benestent II Investigators.J Am Coll Cardiol. 1999;34:1507–1511.
CLINICAL PERSPECTIVE
Among drug-eluting stents released to date, the sirolimus-eluting stent has demonstrated the least amount of late lumen loss, but its efficacy and safety have not been compared head-to-head with the next-generation everolimus-eluting stent. The Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV) trial compared the everolimus-eluting stent with the sirolimus-eluting stent in patients with coronary artery disease. The primary end point was a composite of safety (cardiac death, myocardial infarction, definite stent thrombosis) and efficacy (target vessel revascularization) parameters. A total of 1390 patients were assigned to receive the everolimus-eluting stent and 1384 patients to the sirolimus-eluting stent. At the 9-month follow-up, 4.9% of the patients treated with the everolimus-eluting stent compared with 5.2% of the patients treated with the sirolimus-eluting stent experienced the primary end point (Pfor noninferiority⫽0.01). At the 18-month follow-up, this difference remained. The rate of definite stent thrombosis was higher in the sirolimus-eluting group compared with the eluting group (0.9% versus 0.2%). The everolimus-eluting stent was found to be noninferior to the sirolimus-everolimus-eluting stent.