ZHANG, ZHEN. Structure, Formation, Properties and Relationships in Physically Crosslinked Gels (Under the direction of Dr. Lucian Lucia and Dr. Stephen Michielsen).
by Zhen Zhang
A dissertation submitted to the Graduate Faculty of North Carolina State University
in partial fulfillment of the requirements for the degree of
Doctor of Philosophy
Forest Biomaterials
Raleigh, North Carolina 2019
APPROVED BY:
_______________________________ _______________________________ Lucian A. Lucia Stephen Michielsen
Committee Co-chair Committee Co-chair
ii DEDICATION
iii BIOGRAPHY
iv ACKNOWLEDGMENTS
v TABLE OF CONTENTS
LIST OF TABLES ... viii
LIST OF FIGURES ... ix
1. INTRODUCTION... 1
1.1. Literature review ... 1
1.1.1. Hydrogel formation and classification ... 1
1.1.2. Foam formation and classifications ... 4
1.1.3. A brief review of applications... 7
1.1.3.1. Agriculture ... 8
1.1.3.2. Bio-applications ... 9
1.1.3.2.1. Contact lenses ... 9
1.1.3.2.2. Wound dressings and tissue engineering ... 10
1.1.3.2.3. Drug delivery ... 12
1.1.3.3. Environmental applications ... 13
1.1.3.3.1. Water treatment ... 13
1.1.3.3.1.1. Metal ions and dyes ... 13
1.1.3.3.1.2. Oil/water separation ... 14
1.1.3.3.1.3. Air purification... 15
1.1.3.3.1.4. Other applications ... 16
1.1.4. Characterizations of hydrogels and foams ... 17
1.1.4.1. Water content and swelling parameters ... 17
1.1.4.2. Mechanical properties ... 19
vi
1.1.4.4. Spectrum techniques ... 20
1.1.4.5. Other characterizations ... 21
1.2. Objectives ... 21
2. EXPERIMENTAL METHODS ... 25
2.1. Materials ... 25
2.2. Equipment and sample preparation ... 25
2.3. Characterizations... 26
2.3.1. Water content and swelling/deswelling/reswelling behaviors ... 26
2.3.2. Fourier-transform infrared spectroscopy ... 28
2.3.3. Thermogravimetric Analysis ... 28
2.3.4. Scanning electron microscopy ... 28
2.3.5. Porosity measurement ... 28
2.3.6. Wide-angle X-ray scattering tests ... 29
2.3.7. Differential scanning calorimetry ... 30
3. RESULTS AND DISCUSSION ... 33
3.1. Freeze-dried PVOH gel... 33
3.1.1. Appearance of gels ... 33
3.1.2. Water content and swelling ratio ... 34
3.1.3. Porosity ... 35
3.1.4. TGA tests ... 35
3.1.5. FTIR tests ... 38
3.1.6. DSC studies ... 40
vii
3.1.8. Surface morphology ... 65
3.1.9. Mechanisms ... 66
3.2. PVOH Xerogels ... 71
3.2.1. The deswelling behavior in electrolyte solution ... 71
3.2.2. Rehydrating behaviors ... 80
3.2.3. TGA tests ... 85
3.2.4. DSC studies ... 90
3.2.5. Mechanisms ... 96
3.3. Chitin gels ... 109
3.3.1. Gel appearance ... 109
3.3.2. Water content and swelling ratio ... 110
3.3.3. Surface morphology ... 112
3.3.4. TGA tests ... 114
3.3.5. FTIR tests ... 115
3.3.6. WAXS tests ... 118
3.3.7. Mechanisms ... 119
4. CONCLUSIONS ... 123
5. FUTURE WORK ... 125
viii LIST OF TABLES
ix LIST OF FIGURES
Figure 1.1. Typical examples of physically and chemically crosslinked hydrogels. ... 2
Figure 1.2. Foaming window based on polymer strength and gas/melt equilibrium constant. ... 7
Figure 1.3. A schematic classification of gel applications. ... 8
Figure 1.4. A schematic diagram proposed by Khan and Lo... 14
Figure 1.5. A comparison of stress-strain curves for compression stress (a) and tensile stress (b) of hydrogels. ... 19
Figure 3.1. Physical appearance of hydrogels and foams (from the left to right in digital images: P-H (F), SPP-H (F), CD-H (F), G-H (F) and U-H (F)): Appearance of hydrogels (i), rectangular foams after freeze-drying process (ii), and cone-shape foams after freeze-drying process (iii). ... 33
Figure 3.2. The water content (i) and swelling ratio (ii) of hydrogels and foams. ... 34
Figure 3.3. Porosity of different systems calculated using a solvent uptake method. ... 35
Figure 3.4. Thermal stability of different powders: TGA (i) and DTG curves (ii)... 36
Figure 3.5. A comparison of stress-strain curves for compression stress (a) and tensile stress (b) of hydrogels. ... 37
Figure 3.6. FTIR of different foams: (i) Different natural polymers: (1) SPP; (2) -CD; (3) gelatin; (4) urea; (ii) Different foams: (5) P-F; (6) SPP-F; (7) CD-F; (8) G-F and (9) U-F. ... 39
Figure 3.7. The 1st conventional DSC heating curves of different foams (i-iii) and powders (iv): (1) P-F; (2) SPP-F; (3) CD-F; (4) G-F and (5) U-F; (ii-iii) were the enlarged view of (i). ... 42
Figure 3.8. The 2nd Conventional DSC heating curves (i-ii) and crystallization curves (iii) of different foams: (1) P-F; (2) SPP-F; (3) CD-F; (4) G-F and (5) U-F; (ii) is the enlarged view of (i). ... 43
Figure 3.9. The parameters derived from DSC curves: (i) the 1st heating scan; (ii) the 2nd heating scan; (iii) the cooling scan; (iv) the crystallinity calculated based on different thermal scans; 1H, 2H, and C represented the first heating and the second heating scans and the cooling scan, respectively; (v) the temperature difference between the onset and end of the melting peak. ... 45
x Figure 3.11. The repeated heating cycles of SSA procedures: (i) P-F; (ii) SPP-F; (iii)
CD-F; (iv) G-F and (v) U-F. ... 47 Figure 3.12. The parameters derived from SSA curves: (i) relationship between Tm and Ti;
(ii) melt enthalpy of different “fingers” versus temperature; (iii) melt enthalpy fractions versus lamellar thickness; (iv) melt enthalpy derived from SSA peaks and the whole curve (the blunt endothermic peak before different thermal fractionations was also included). ... 48 Figure 3.13. DSC curves of ice-water transition of different hydrogels (i): (1) P-H; (2)
SPP-H; (3) CD-H (4) G-H and (5) U-H, and enthalpy of the transition (ii). ... 50 Figure 3.14. MDSC/DSC curves of different hydrogels: (i) Total heat flow MDSC curves;
(ii) non-reverse MDSC heat flow curves; (iii) reverse MDSC heat flow curves; (iv) DSC curves of P-H. ... 53 Figure 3.15. The MDSC curves under heat-only condition (2 oC/min): (i) P-F, (ii) SPP-F;
(iii) CD-F; (iv) G-F; (v) U-F; the solid, dash and dot curves represent the total heat flow, the reverse heat flow and the non-reverse heat flow curves, respectively. ... 55 Figure 3.16. The MDSC curves under heat/cool condition (2 oC/min): (i) P-F, (ii) SPP-F;
(iii) CD-F; (iv) G-F; (v) U-F; the solid, dash and dot curves represent the total heat flow, the reverse heat flow and the non-reverse heat flow curves, respectively. ... 56 Figure 3.17. The MDSC curves under heat/cool condition (3 oC/min): (i) P-F, (ii) SPP-F;
(iii) CD-F; (iv) G-F; (v) U-F; the solid, dash and dot curves represents the total heat flow, the reverse heat flow and the non-reverse heat flow curves, respectively. ... 57 Figure 3.18. The reversing, non-reversing and the total melt enthalpy derived from MDSC
curves: (i) Heat-only condition (2 oC/min); (ii) heat/cool condition (2 oC/min); (iii) heat/cool condition (3 oC/min). ... 60 Figure 3.19. The melting peak temperature of different MDSC curves; the R, NR, T in the
inserted legend represented reversing, non-reversing and the total heat flow curves, respectively; 1, 2, and 3 corresponded to heat-only condition (2 oC/min), heat/cool condition (2 oC/min), and heat/cool condition (3 oC/min), respectively. ... 61 Figure 3.20. Wide angle X-ray scattering (WAXS) curves of powder (i) a-pure PVOH
xi Figure 3.21. Crystal parameters derived from WAXS curves: crystallinity was based on the
peak at 2=19o; peak I and peak II referred 2=19o and 2=40o, respectively; powder represented pure PVOH powder. ... 64 Figure 3.22. Surface morphological results of different foams (rectangular shape): (i) P-F;
(ii) SPP-F; (iii) CD-F; (iv) G-F; (v) U-F. ... 65 Figure 3.23. A schematic representation of the process from hydrogels to foam. ... 66 Figure 3.24. PVOH crystallites density calculated based on the melt enthalpy measured by
traditional DSC (i) and crystallinity measured by WAXS (ii). ... 68 Figure 3.25. Schematic representation of freeze-drying process of (i) CD-F and U-F and
(ii) SPP-F and G-F; Lamellar thickness of PVOH crystallites density of different systems (iii). ... 70 Figure 3.26. Effect of electrolyte concentration on the weight loss of different hydrogels:
(1) P-H; (2) SPP-H; (3) CD-H; (4) G-H; (5) U-H. ... 73 Figure 3.27. Effect of electrolyte concentration on the WRL of different hydrogels: (1)
P-H; (2) SPP-P-H; (3) CD-P-H; (4) G-P-H; (5) U-H. ... 74 Figure 3.28. Effect of electrolyte concentration on the WRL of different hydrogels on the
initial stage: (1) P-H; (2) SPP-H; (3) CD-H; (4) G-H; (5) U-H. ... 75 Figure 3.29. Effect of electrolyte concentration on the CI of different hydrogels: (1) P-H;
(2) SPP-H; (3) CD-H; (4) G-H; (5) U-H. ... 76 Figure 3.30. Effect of electrolyte concentration on the WRI of different hydrogels: (1)
P-H; (2) SPP-P-H; (3) CD-P-H; (4) G-P-H; (5) U-H. The WRI value at 1800 minutes was selected as the raw data for plotting. ... 78 Figure 3.31. Effect of electrolyte concentration on the CI of different hydrogels: (1) P-H;
(2) SPP-H; (3) CD-H; (4) G-H; (5) U-H. The CI value at 1800 minutes was selected as the raw data for plotting. ... 80 Figure 3.32. The recovery efficiency of different gel systems after the reswelling process:
1, 2, 3, 4, and 5 represented P-H, SPP-H, CD-H, G-H and U-H, respectively. xM-yh, means the fresh hydrogels treated in x M NaCl solution for y hours before drying and reswelling; while xM-E means the samples were treated in x M NaCl solution for 3 days before drying and reswelling. ... 82 Figure 3.33. The digital image of xerogels before being reswollen (a) and reswollen
xii In (b), the samples were fresh hydrogels, and reswollen hydrogels in 0M, 1M,
2M and 3M NaCl solutions, respectively, from the top to the bottom. ... 83 Figure 3.34. Effect of electrolyte concentration on the CI of different hydrogels: (1) P-H;
(2) SPP-H; (3) CD-H; (4) G-H; (5) U-H. The CI value at 1800 minutes was selected as the raw data for plotting. ... 84 Figure 3.35. TGA curves of different materials: (i) P-X; (ii) SPP-X; (iii) CD-X; (iv) G-X;
(v) U-X. ... 85 Figure 3.35. DTG curves of different materials: (i) P-X; (ii) SPP-X; (iii) CD-X; (iv) G-X;
(v) U-X. ... 86 Figure 3.37. The initial degradation temperature of different systems: (i) the temperature
at 10 wt% weight loss; (ii) the temperature at 20 wt% weight loss. ... 87 Figure 3.38. The weight loss of different degradation steps of different systems: (i) P-X;
(ii) SPP-X; (iii) CD-X; (iv) G-X; (v) U-X (the weight of moisture loss (the 1st
degradation step) was not included in the calculation). ... 88 Figure 3.39. DSC first heating curves of different materials: (i) P-X; (ii) SPP-X; (iii)
CD-X; (iv) G-CD-X; (v) U-X. ... 92 Figure 3.40. DSC second heating curves of different materials: (i) P-X; (ii) SPP-X; (iii)
CD-X; (iv) G-X; (v) U-X. ... 93 Figure 3.41. DSC cooling curves of different materials: (i) P-X; (ii) SPP-X; (iii) CD-X; (iv)
G-X; (v) U-X. ... 94 Figure 3.42. Parameters derived from DSC curves of different systems: (i) melt enthalpy
of the 1st heating curve; (ii) crystallization peak temperature; (iii) crystallization enthalpy; (iv) melt peak temperature of the 2nd heating curve; (v) melt enthalpy of the 2nd heating curve. ... 95 Figure 3.43. The weight difference of electrolyte solution-treated xerogels before and after
being reswollen in DI water. ... 97 Figure 3.44. The relationship between recovery efficiency and enthalpy values: the black
rectangular, red cycle, blue up triangle, pink down triangle, and olive star symbols represented P-X, SPP-X, CD-X, G-X and U-X, respectively; the symbol filled with solid color, white color, and cross referred the enthalpy of the 1st heating, cooling, and the 2nd heating scan, respectively. ... 101 Figure 3.45. The relationship between recovery efficiency and lamellar thickness
xiii triangle, and olive star symbols represented P-X, SPP-X, CD-X, G-X and
U-X, respectively; the symbol filled with solid color, white color, cross and the horizontal line referred different xerogels treated by 0M, 1M, 2M and 3M electrolyte solution, respectively. ... 105 Figure 3.46. The schematic representation of the mechanism for the xerogels with different
swelling capability: xerogel-X, xerogel-X-NaCl and Hydrogel-X represented the xerogels treated by DI water, NaCl solution and the reswollen hydrogels, respectively; I, II, III and IV represented PVOH, VOH/CD, PVOH/natural polymers (SPP and gelatin), and PVOH/urea systems, respectively. ... 107 Figure 3.47. Physical appearance of the as-prepared hydrogels (a) and solid foams (b): the
digital image of hydrogels from the left to the right are pure chitin hydrogels prepared from 3 wt% and 9 wt% chitin suspension, respectively; the digital image of chitin solid foams from the left to the right are pure chitin foams generated from 3 wt%, 3 wt%, and 9 wt% chitin hydrogels after dialysis process, respectively. ... 109 Figure 3.48. Water content (i), SR versus swelling time during the 1st swelling process (ii),
and SR versus swelling time during the 2nd swelling process (iii) of the hydrogels and solid foam. ... 111 Figure 3.49. Surface morphology of different solid foams (at 3000 times magnification):
(i) and (iii) refer different morphological results of UD-3wt%; (ii) and (iv) represent different morphological results of UD-9wt%; (v) and (vi) is corresponded with the morphology of D-3wt% and D-9wt%, respectively. ... 112 Figure 3.50. Surface morphology of different solid foams (at 10000 times magnification):
(i) and (iii) refer different morphological results of UD-3wt%; (ii) and (iv) represent different morphological results of UD-9wt%; (v) and (vi) is corresponded with the morphology of D-3wt% and D-9wt%, respectively. ... 113 Figure 3.51. TG and DTG results of different chitin foams: (i) TG curves; (ii) DTG curves;
(iii)-(iv) Different parameters derived from TG and DTG curves; T10%, T20% and T50% referred the temperature at the weight loss of 10, 20 and 50 wt%, respectively; the missing data of T50% of Chi-UD3 and Chi-UD9 was because of their residue mass were higher than 50 wt%. ... 114 Figure 3.52. (i) FTIR curves: a, b, c, d, and e represented chitin powder, D-3wt%, D-9wt%,
UD-3wt%, and UD-9wt%, respectively; (ii) Relative peak intensity ratio of different characterization peaks in different materials. ... 116 Figure 3.53. WAXS results of different chitin materials: (i) XRD curves of different
samples (a, b, c, d, and e represent the pure chitin powder, U3wt%,
xiv Figure 3.54. Half a year static alkali treatment of chitin suspension at room temperature;
from the left vial to the right vial, it represented chitin-3wt% and chitin-9wt%, respectively. ... 120 Figure 3.55. A schematic representation of the gel formation mechanism: (I) A simplified
1 1. INTRODUCTION
1.1. Literature review
1.1.1. Hydrogel formation and classification
With the development of the step-growth polymerization and crosslinking mechanisms for thermosets [1], a sturdy base for preparing hydrogels was provided. Hydrogel is a wet material that can hold a large amount of water, which is several times of its dry weight [2]. Three-dimensional (3D) networks within hydrogels not only help to imbibe water, but also support the hydrogels as the “bones” because of their crosslinking points (the network lattice joints) [3]. Because of these unique features, hydrogels are usually considered as an intermediate state between solid and liquid, that is, it can keep the viscous and elastic characters simultaneously.
3 From the perspective of hydrogel formation mechanisms, it has two categories: one is chemically crosslinked hydrogel while the other is physically crosslinked hydrogel. Figure 1.1 briefly summarized the classifications of physically and chemically crosslinked hydrogels in a recent review paper [3]. Typical chemically crosslinked hydrogel is prepared by introducing the covalent bonds between different polymeric chains. In this case, the original materials of hydrogel can be either reactive monomers (small molecules) or polymers. After employing different strategies (i.e., adding crosslinkers, ultraviolet light or -beam irradiation) or different types of chemical reactions (i.e., esterification, Michael addition, Schiff’s base formation), the covalent bonds are permanently formed [3]. For crosslinking reactions, the crosslinking density strongly depends upon different parameters such as the crosslinker dosage, the initiator for the polymerization, the wettability of the monomers, and the reaction temperature [5]. The crosslinker must be multifunctional (f ≥ 2) so that it can react and connect different polymeric chains [1]. Chemical-crosslinked hydrogels are of irreversible nature and cannot be dissolved by water, even though a large portion of them are still of hydrophilic nature. In addition, the heat input cannot melt the polymeric content in the wet hydrogel under heating condition that is similar with rubber and thermosets; but water evaporation will occur inevitably [1].
4 Besides adding crosslinkers (i.e., borax in slime systems), physical-crosslinked hydrogels can also be prepared via other methods such as freeze-thawing technique. One typical example is poly (vinyl alcohol) (PVOH) hydrogels prepared by repeated freeze-thaw cycles [9]. This will either help to reduce the interchain distance and thereby form more hydrogen bonding, or promote the growth of small crystallites, where they can serve as the physical crosslinkers [9]. Compared with the chemical crosslinked hydrogels, the external stimuli (e.g., heat) can break the physical crosslinking points into sol state [10]. In addition, some physically crosslinked hydrogels can be dissolved in water. The properties of physically crosslinked hydrogels can be tailored by controlling different experimental parameters such as the polymer concentration, charge density of solution, pH, ionic strength and other factors [2, 3].
1.1.2. Foam formation and classifications
Solid foam, also termed as “sponge”, is a kind of dual-phase material: gaseous voids serve as the dispersed phase and is located within a dense continuous phase [11]. Solid foams have several different kinds of applications from natural to industrial products. For polymeric foams, its unique physical properties are determined by both the properties of the polymeric matrix, the air phase, and the cellular structures [12]. The polymeric skeletal frame supports the strength; while the air phase and the porous structures of foams offer the foams with special merits. For example, the low thermal conductivity of air makes the foams widely used as thermal insulators [13]; while the porous structure and high surface area endows the foams with high potential for surface-modification and functional applications (i.e., adsorption of water pollutants [14]).
5 during the polymerization. A typical example is polyurethane (PU) foam, where the isocyanate (one of the monomers for the polymerization of PU) can react with water to give rise to carbon dioxide and thereby participate in the foaming process [11]. In addition, the decomposition of chemical blowing agent (CBA) is also an important sub-category to prepare a foam. On the other hand, soluble foaming is a crucial methodology for preparing polyolefin foams (it occupies 38% of market [11]). In this case, a melt extrusion is introduced to continuously produce foams via introducing physical blowing agent (PBA) into the polymeric matrices. During the melt-mixing process, the PBA (usually in gaseous state) can be dissolved in the polymeric melt to form a homogenized polymeric melt/gas binary hybrid system. With the further assistance of low-pressure condition, the foaming can occur [11]. The third methodology is derived from the way of preparing an aerogel [11]. In this method, a polymer solution is utilized, and the solvent herein serves as the blowing agents. Unlike the afore-mentioned two methodologies, where the blowing agents served as the minor phase, the solvent herein serves as the major phase while the polymer chains work as the skeleton. In this case, different polymer chains will be inter-connected via either physical interactions (e.g., chain entanglements, hydrogen bonding) or covalent bonds. After the solvent is removed from the skeleton via different drying method (e.g., vacuum heating, supercritical drying), the foam can be obtained. It should be noted that foams prepared via this strategy are not well developed and have limitations because of its high expense of facilities and lack of continuous production capacity. In this case, they are of higher academic interest than of practical/industrial interest.
6 based on their different rigidity. Based on their morphological results (cellular structures), the foams can be categorized as “open-cell foams” and “close-cell foams”. The foams can also be distinguished between the blowing agents they used (either chemical or physical blowing agents).
7 Figure 1.2. Foaming window based on polymer strength and gas/melt equilibrium constant [11].
1.1.3. A brief review of applications
8 Figure 1.3. A schematic classification of gel applications [16].
1.1.3.1 Agriculture
9 swelling ratio value is higher than 100) [19], which makes them ideal candidates as nutrient carriers. There are two ways to load the nutrient in the hydrogels, including post-loading (the process of loading nutrient is finished after the formation of hydrogel) and in-situ-loading method (the process of loading nutrient and hydrogel formation is finished at one-pot process) [17]. The nutrition-loaded hydrogels will be added into the soil, and then be swollen in the rainy season, during which some of the nutrient will be released and diffused into the soil; while during the rainy season, some nutrient is preserved and will be released in future. A series of fertilizers/nutrients such as herbicide paraquat [20], neem (Azadirachta Indica A. Juss.) seed oil [21], 2,4-dichlorophenoxyacetic acid [22], urea [23], nitrogen fertilizer [24] have been reported. Different hydrogel systems both from synthetic polymers to polysaccharides and minerals have been selected as the loaded matrices [17]. In these studies, the structure-properties relationship as well as the swelling and releasing kinetics were fully elucidated. Although these studies demonstrated that SH/modified-SH have great potential to be applied in agricultural fields, there is still a long way to commercialize SH/modified-SH for large scale operations [25] because of the high final cost of production and their sensitivity to salts.
1.1.3.2. Bio-applications 1.1.3.2.1. Contact lenses
10 patented. Hydrogels, including poly-2-hydroxyethylmethacrylate (PHEMA) and silicone (silicone-containing segment derived from polysiloxane linked with hydroxyl or amino groups) hydrogels, have been developed as soft contact lenses according to a recent review [26]. For PHEMA hydrogels, a lot of attempts were reported to offer this hydrogel system with better physical and chemical performances. Neefe [30] attempted to employ small light reflecting particles to tailor the optical properties of hydrogels. Other attempts such as reinforcing/strengthening purpose via copolymerizing HEMA with some hydrophobic monomers and resisting the microbial growth by adding antibacterial agents have also been reported [31]. With respect to silicone hydrogels (SiHy), even though they have advantages like excellent oxygen permeability and have become the most popular one in the market, its wettability causes the protein deposition and thereby results in protein spoilage [26]. To increase the surface wettability, plasma treatment [32], and bio-mimic nano-textured surface [33] have been reported. Besides the vision applications, these soft contact lenses can be modified to overcome the low drug loading capacity and the burst releasing rate and thereby applied for drug delivery to eye [34]. Hu et.al. [34] reviewed several methods such as hydrophilic/hydrophobic copolymerization, colloid-laden hydrogels with the assistance of nanoparticles, and preparing ligand-containing hydrogels with the assistance of cyclodextrin. Thereby, developing functional hydrogels not only in target of contact lenses but also in target of extended ophthalmic drug delivery will be of strong desire and great importance in future.
1.1.3.2.2. Wound dressings and tissue engineering
12 1.1.3.2.3. Drug delivery
One of the most important bio-applications of hydrogels is drug delivery. Many academic papers regarding drug delivery application of hydrogel have been published; however, only a few finally became commercial products. Similarly to fertilizer-loaded hydrogels for agriculture, hydrogels in drug delivery can either be loaded in the dry state via immersing the gel in the drug solution or via the so-called “in-situ gelling”, where the crosslinking and loading occur simultaneously [37]. The latter is of more significance in clinical usage since the gelled ones has no flowability (the crosslinking nature of hydrogels hinders the chain motion) and could not be injected to the targeted areas. Because of the high water content of the hydrogels, the loaded drugs are easily released from the hydrogel matrix in hours or days, which is shorter than that of other drug carriers [37]. To fix this problem, several strategies such as preparing interpenetrating polymer network (IPN), surface diffusion control, incorporation of micro-particles and micelles have been reported [38-40]. The idea of these techniques is to introduce some structures to entrap the drugs and therefore prevent their fast release rate.
13 1.1.3.3. Environmental applications
1.1.3.3.1. Water treatment 1.1.3.3.1.1. Metal ions and dyes
14 factor that determines the kinetics. Similar as the diffusion kinetics discussed previously, higher crosslinking density usually results in lower swelling ability. As a result, the adsorption efficiency will be decreased.
Figure 1.4. A schematic diagram proposed by Khan and Lo [53].
For further trend and challenges, the attention of research paper will be changed from science (adsorption capacity and kinetic modeling (i.e., Langmuir adsorption model)) to engineering (commercial scale rather than lab scale). From this viewpoint, how to reuse/regenerate the hydrogels, reutilize/recycle the adsorbed pollutants to valuable chemicals, and designing scalable engineering units will be fixed by both chemist and chemical engineers (see Figure 1.4) [53]. Other important challenges such as the cost of the raw materials, suitable facility of preparation the crosslinking of hydrogels will also need to be addressed in future.
1.1.3.3.1.2. Oil/water separation
15 (i.e., graphene oxide foam [56], carbon nanotube foam [57]). For oil/water separation, it needs the surface of the foam/sponge/aerogel to be super-hydrophobic and super-oleophilic. Therefore, much scientific interest has been placed onto the surface modification of these foams [58]. A variety of methods, including in situ growth method, dip-coating method, chemical vapor decomposition (CVD) and surface modification techniques have been widely used [59-62]. No matter what kinds of technique is used, the main idea is to create a hydrophobic layer (i.e., silane layer) on the surface of foam/sponge/aerogel, which could expel the water but absorb oil. To characterize the separation capacity, the absorbing capacity (grams of oil/gram of absorbent) and surface wettability (contact angle tests) was usually utilized. Besides the absorbing capacity, the reusability of the foams/sponges/aerogels (similar like the reusability mentioned in the last section) was evaluated in many papers [48, 53]. The used foam/sponge/aerogel was pressed to extract the absorbed solvents, followed with/without the chemical treatment to dissolve the oil residue to ultimately retest the absorbent efficiency. There are still some further challenges that restrict this application. For example, some rigid foams cannot be pressed to extract the oil pollutant because of their high stiffness. For some foams with low porosity, it is difficult for surface modification to penetrate the inner part of the foams. As a result, the super-hydrophobic and super-oleophilic properties is only restricted to the surface. In this case, the foams will not work effectively as an absorbent if the surface layer is broken or worn out.
1.1.3.3.1.3. Air purification
16 capture of greenhouse gas-carbon dioxide (CO2) and volatile organic compounds-BTEX (benzene, toluene, ethylbenzene, and xylene) according to a recent review [63]. For the capture of CO2, the introduction of amine group onto the surface of aerogel is very effective to absorb CO2, where CO2 molecules can react with amine groups to form ammonium carbamate [64]. The amine groups can be introduced by either physical interactions (i.e., dip-coating method [65]) or covalent bonds (i.e., via grafting aminotrialkoxysilanes onto a silica surface [66]). The source of amine groups can come from either small molecule (i.e., aminotrialkoxysilanes [66]) or macromolecules (polyethyleneimine (PEI) [67]). It should be noted that the introduction of these amine groups inevitably causes decrease in the surface area of the aerogels [68], even though this issue does not influence the absorption of CO2. Regarding the adsorption of BTEX, carbon aerogels [69] have been reported to effectively absorb toluene vapor together with great feasibility of regeneration. Silica aerogel is effective to remove the benzene vapors and further surface-modification has been proved to be of great importance for the reusability of aerogels because of the increased hydrophobicity [70]. In future, it is envisioned that utilization of the aerogels for the indoor purpose of air filtration (i.e., remove the PM2.5 and other hazardous particles [71]) will receive more and more interest for both academic and industrial fields. However, how to commercialize aerogel at large scale (i.e., the reusability of aerogels and the cost for the preparation of aerogels) will be challenged.
1.1.3.3.1.4. Other applications
17 while simultaneously it can also work as functional additive to other systems to endow the matrix with electrically conductive functions [73]. In addition of the electronic applications, other areas such as photo-luminance [74] and catalyst [75] have also been reported. There is one important classification of hydrogels named with “stimuli-responsive” hydrogels. In this case, the hydrogels can suddenly get/lose its functionality when external stimuli (i.e., temperature, pH, magnetic field) is placed/unloaded on the hydrogels [76]. This kind of hydrogel with the tunable properties can simulate nature (bio-inspired/bio-mimic) and thereby work as advanced intelligent materials. Although these materials show great potential in different fields, how to commercialize them at large scale is still challenging and needs to be fixed in future.
1.1.4. Characterizations of hydrogels and foams 1.1.4.1. Water content and swelling parameters
There are several parameters, including water content, swelling ratio, water uptake and water retention, that characterize the water. In general, these parameters are related to either the hydrogels (wet state) or foams/films (dry state). For water content (WC), it is defined as the fraction of water contained in the fresh hydrogels at the room temperature, and it can be calculated as follows: 100% f d f W W WC W −
= (1)
where Wf and Wd refer the weight of fresh and dried gels.
For swelling ratio (SR), its definition is still unclear. In some work [19], the SR just refer as the maximum capacity of holding water and can be calculated based on the following calculation:
100% s d W SR W
18 where Ws and Wd refer the weight of the hydrogel at the equilibrium swelling state and dried gel, respectively.
The SR is a fixed value and the equilibrium state refers the state that the fresh hydrogel is further immersed into water for long period where the equilibrium is built up. In these papers, the water uptake (WU) was used to record the swelling kinetics of the dried gels:
100% t d d W W WU W −
= (3)
where Wt and Wd refer the weight of the hydrogel at time t and the dried gel, respectively. However, sometimes SR is not a fixed value and plays a similar role as WU does:
100% t d d W W SR W −
= (4)
where Wt and Wd are the same symbol as proposed in equation 3.
Finally, water retention (WR) that is useful for deswelling kinetics study can be calculated as follows: 100% t d s W W WR W −
= (5)
where Wt, Ws and Wd are the same as Equations (2)-(4).
19 biomedical applications. It should be noted that different papers have tested these parameters in their own ways. For example, the “Wd ” can be measured via drying the hydrogels in hot oven, vacuum oven, freeze-drier, and supercritical drier. Some authors use a tea bag to wrap the gel while some papers directly place the gel into the tested solvent. All these may bring experimental errors determined by their different capacity of removing water and moisture residue.
1.1.4.2. Mechanical properties
Figure 1.5. A comparison of stress-strain curves for compression stress (a) and tensile stress (b) of hydrogels [77].
20 of the foams are more complicated due to different factors (i.e., porosity, open/close cells). Whether tensile or compressive tests will be selected strongly depends upon the physical state of hydrogels. For example, if the sample cannot bear its weight/gravity or the sample is easy to flow, it might not be accurate for either test. Other mechanical test such as creep behavior has also been reported [78].
1.1.4.3. Thermal properties
Thermal characterizations, including differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), can be utilized to characterize the pre-dried gels (foams/sponges/aerogels). Depending upon the nature of polymers and the employed crosslinking chemistry, different pieces of information such as glass transition temperature, melting and crystallization temperature can be obtained in DSC curves. The information of thermal stability, degradation rate and char content can be obtained from TGA. These different thermal analysis characterizations are usually placed upon the pre-dried gel samples (foams/sponges/aerogels/films) rather than hydrogels.
1.1.4.4. Spectrum techniques
21 1.1.4.5. Other characterizations
There are a lot of other characterizations available for hydrogels and foams/sponges/aerogels. Rheometer can be used to record the gelation/crosslinking process of the hydrogels, offer the gel point, and simulate the self-healing behaviors [81]. The microscopies such as scanning electron microscopy (SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM) are effective to characterize the porosity, the cell structures, different phase structures and surface morphologies of foams/sponges/aerogels. Because of the invention of cryo-SEM, it may be useful to record the thawing process of the frozen hydrogels in future. Other characterizations like dynamic mechanical analysis (DMA), thermal conductivity, Brunauer–Emmett–Teller (BET), and thermal mechanical analysis (TMA) are also important characterizations.
1.2. Objective
Recently, there has been significant research interest on the post-processing of hydrogels using either supercritical drying or freeze-drying to sublimate water while simultaneously maintaining 3D network structural memory. The resultant product is of high porosity (80%-90%) and foam-like in appearance [63]. These highly porous materials with/without further surface modification have been used as energy devices, water treatment systems, in air purification, as catalysts, thermal insulation and other applications as discussed previously [63]. However, one key problem in freeze-drying is dimensional shrinkage and structural collapse of the foam tend to occur. Such substantial structural damage leads to pore dysfunctionality, and therefore compromises their performance as porous materials.
22 shrinkage) compared to gels prepared by sublimation termed “xerogels” [82]. Because of their compacted structure, xerogels are difficult to rehydrate, a limitation that restricts their capacity to be loaded with drugs for further delivery application.
23 The above theories focused on PVOH hydrogel formation with respect to water freezing. Composite structures as shown in nature provide useful physico-chemical properties that can be summative or synergistic relatively to the components. In this dissertation, the influence of natural polymers, including soy protein polymer and gelatin on the gel formation of PVOH were investigated. It was interestingly found that the addition of these two natural polymers promoted the appearance of the foam formation during sublimation, while improving the reswelling/rehydrating capacity of oven-dried gels compared to pure PVOH. In addition, -CD and urea were also selected to see whether they could prevent or negatively influence hydrogel and solid foam formation. More specifically, for -CD, its hydroxyl groups can participate in specific interactions such as hydrogen bonding although it has been reported that -cyclodextrin (-CD) can negatively influence the hydrogel formation of poly (vinyl alcohol) [88]. For urea, it has been reported that urea can induce hydrogen bonding between its carbonyl group and the hydroxyl groups in poly (vinyl alcohol) and effectively decrease its crystallization ability [89]. In this dissertation, it was found that both -CD and urea failed to promote solid foam formation and reswelling performance of xerogels. Based on these results, it was attempted to explain the mechanisms of improved solid foam formation and rehydrating behaviors. In addition, the structure and properties of different gels were studied. The deswelling behaviors of different hydrogels in salt/electrolyte solutions were also investigated.
25 2. EXPERIMENTAL METHODS
2.1. Materials
All the materials used in this dissertation were commercially accessible. Poly (vinyl alcohol) (PVOH) was purchased from Sigma-Aldrich at a hydrolysis degree of 99%. Soy protein polymer (SPP, SobindTM) was provided by Dupont. Gelatin (S25335) was obtained from Fisher Scientific. Urea was obtained from Sigma-Aldrich. -cyclodextrin (-CD) was a gift from Wacker. Chitin powder from shrimp shell with a yellowish color was purchased from Sigma-Aldrich. Sodium hydroxide (NaOH) was provided by Sigma-Aldrich. Sodium chloride (NaCl, ACS reagent, ≥99%) was purchased from Sigma-Aldrich. 1-Butanol (99+%, extra dry, AcrosealTM) was provided by ACROS Organics. DI water was obtained from a reverse osmosis system and used as the basic solvent for preparing different hydrogels and solutions/suspensions.
2.2. Equipment and sample preparation
The chemical reaction supporting systems, freezer, freeze-drier and hot oven were all available. Other instruments, such as differential scanning calorimetry (DSC), thermogravimetric analyzer (TGA), scanning electron microscopy (SEM) and Fourier-transform infrared spectroscopy (FTIR) and X-ray Diffraction (XRD) diffractometer were all accessible.
26 PVOH/urea were denoted as P-i, SPP-i, CD-i, G-i and U-i, respectively. The “i” herein can be either “F”, “H”, and “X” that represented solid foam, hydrogel and xerogel, respectively. The solutions were poured into different molds and tubes. Different PVOH hydrogels were prepared using successive freeze-thaw (FT) cycles in a freezer for ~ 17 hr and thawed at room temperature for 7 hr. Finally, the as-prepared hydrogels exhibited homogeneous appearance. To convert the hydrogels into foams, they were freeze-dried ~ 5 days under 0.110 mBar. To prepare xerogels, hydrogels were dried in on oven at 60 oC for ~ 6 hours until their weight did not change. To evaluate the effect of concentration of the electrolyte on the deswelling/reswelling behaviors of PVOH hydrogels, three different concentrations (1M, 2M, and 3M) of sodium chloride (NaCl) solution were selected. These different NaCl solutions were prepared by mixing solid NaCl crystals with DI water.
For the preparation of chitin hydrogels, pre-weighted chitin powder and NaOH solution (6.6 wt%) were mixed together and transferred to a plastic mold. After a 5-min mixture, the mold was immediately transferred to a freezer for 17 hrs. The mold was picked up from the freezer and thawed at room temperature. Yellowish hydrogels were formed, and some were further dialyzed. Both the un-dialyzed and dialyzed hydrogels were freeze-dried to obtain solid foams. For simplification, the un-dialyzed and dialyzed chitin hydrogels were denoted as UD-x wt% and D-x wt%, where UD, D and x represented the un-dialyzed sample, dialyzed sample and the chitin weight fraction, respectively.
2.3. Characterizations
2.3.1. Water content and swelling/deswelling/reswelling behaviors
27 1. The weight before and after drying was recorded and used for the calculation. The swelling behavior was detected by immersing the dried samples into DI water for a specific time interval. For each measurement, the surface water of the swollen sample was blotted softly by wiper paper [103]. The swelling ratio (SR) was defined as the ratio between the water uptake (the difference between the wet weight and dry weight) and dry weight of each sample.
The water desorption (deswelling) behaviors of different PVOH-related hydrogels in electrolyte (NaCl) solution were studied. The fresh hydrogels were directly immersed into electrolyte solution. At a certain time interval, the hydrogel samples were taken out from the solution and water was wiped from the gel surface, and the gel was weighed. The weight loss of the hydrogels was weighed as a function of immersing time. To better quantitatively express the deswelling behaviors, two parameters, the water retention loss (WRL) and the collapse index (CI), were proposed as follows:
𝑊𝑅𝐿 = 𝑚𝑡−𝑚𝑑
𝑚0−𝑚𝑑 (6)
𝐶𝐼 =𝑚0
𝑚𝑡 (7)
where m0, mt and md represents the mass of the hydrogel before testing, the hydrogel mass at time t, and the mass of the xerogel (dried in a hot oven at 60 oC), respectively.
28 2.3.2. Fourier-transform infrared spectroscopy
Fourier-transform infrared spectroscopy (FTIR,CW General Purpose Mid-IR Bruker Alpha) was used to observe possible interactions between different components. All the tests were carried out at 32 scans and 4 cm-1 resolution.
2.3.3. Thermogravimetric Analysis
TGA tests of different foams were carried out in a TGA analyzer (TA Q500). Each thermo program was set up over a temperature range of 40-600 oC and heating rate of 10 oC/min. The sample weight was ~ 5-10 mg while a nitrogen atmosphere was applied to prevent thermal oxidation. For comparison purposes, pure powders (e.g., PVOH, SPP, -CD, gelatin, urea, chitin) were tested under the same testing conditions.
2.3.4. Scanning electron microscopy
Scanning electron microscopy (SEM, JSM-6360LV) was used to investigate the surface morphology of the rectangular PVOH and chitin solid foams. Before observation, the sample surface was coated with a thin layer of gold. An accelerating voltage of 20 kV was utilized. No chemical or any other etching/staining treatment was placed upon the samples.
2.3.5. Porosity measurement
A solvent uptake method was utilized to determine the porosity. Specifically, the following equation was applied:
𝑃𝑜𝑟𝑜𝑠𝑖𝑡𝑦 (%) = (𝑊2−𝑊1+a∗𝑊1)𝜌1
29 where W1, W2, 1 and 2 represents the initial foam weight, swollen foam weight, the density of solid parts of the solid foam and the density of i-butanol (0.8 g/cm3), respectively. To obtain
1
and a, equations 9-10 were used, respectively: 1
𝜌1 =
𝑤1
𝜌PVOH+
𝑤2
𝜌2 (9)
where 𝑤1, 𝑤2, 𝜌1,𝜌𝑃𝑉𝑂𝐻 and 𝜌2 represent the weight fraction of PVOH, weight fraction of the second component, the density of solid parts of the foam, PVOH (1.19 g/cm3), and the second component, respectively. For calculation of 𝜌1, the 𝜌2 value of soy protein, -cyclodextrin, gelatin, and urea were 1.30 g/cm3, 1.44 g/cm3, 1.27 g/cm3 and 1.32 g/cm3, respectively. The calculated 𝜌
1
for PVOH/SPP, PVOH/-CD, PVOH/gelatin and PVOH/urea binary systems were 1.221 g/cm3, 1.192 g/cm3, 1.213 g/cm3, and 1.191 g/cm3, respectively.
𝑎 = 𝑤1∗ 𝑎1+ 𝑤2∗ 𝑎2 (10)
where 𝑤1, and 𝑤2 referred to the weight fraction of PVOH and the 2nd component system; To determine the 𝑎1 and 𝑎2 value of different systems, a film of PVOH, SPP, and gelatin (They are 6wt%, 2wt%, and 2wt% DI water solution/suspension in order to simulate the similar condition in the solid foam) was casted firstly. Then, portions of the films were immersed into i-butanol to observe weight change. It was found that the weight change in PVOH, SPP and gelatin in reference with the sample weight before immersion was 0.0243, -0.0117, and -0.0498, respectively, which corresponded to an 𝑎1 value of PVOH and 𝑎2 values of SPP and gelatin. For -CD and urea, their
𝑎2 values were 0 and 0.01, respectively. 2.3.6. Wide-angle X-ray scattering tests
30 PVOH and chitin solid foams. Diffraction patterns were scanned from 10 to 40° at a scanning rate of 10°/min. In order to investigate crystal structures, the apparent crystallite size (Lhkl) was calculated according to the Scherrer formula [104]:
Lhkl=kλ/Bcosθ (11)
where λ was 0.154 nm, θ is the Bragg angle, k=0.89 was the structure factor, and B was taken as the full width at half-maximum of a certain diffraction peak.
2.3.7. Differential scanning calorimetry
Both traditional/conventional DSC and modulated DSC (MDSC) modes were used to characterize the PVOH hydrogels and foams. All DSC and MDSC for hydrogels were completed as quickly as possible to rule out ageing; thus, all of them were finished within one day after being prepared. For the hydrogels, DSC was first used to study ice-water transitions within the hydrogels. For each test, a sample of 10-20 mg was first equilibrated at -30 oC and scanned to 30 oC at a scanning rate of 5 oC/min. For each system, it was tested at least twice to guarantee that the difference between different values of melt enthalpy were within 15 J/g and the average values were taken as the results. MDSC was used to determine the enthalpy of gel-sol transition. Each sample was first equilibrated at 40 oC and heated to 95 oC at a heating rate of 1 oC/min with an amplitude of 1 oC and period of 60 s. All the DSC/MDSC tests were carried out under nitrogen atmosphere (gas purge rate = 50 ml/hr).
31 ml/hr). Crystallinity is the quotient between the observed enthalpy and the value of 138.6 J/g (the enthalpy value of melting of 100% crystalline PVOH) and the weight fraction of PVOH. MDSC was also utilized to characterize foams. Each sample of 8-10 mg was heated to 250 oC. Three different modulation and testing conditions were used: 3 (oC/min)/amplitude of 1 (oC)/period of 60 s, 2 (oC/min)/amplitude of 1 (oC)/period of 60 s, and 2 (oC/min)/amplitude of 0.32 (oC)/period of 60 s. The last one was classified as “heat-only” condition, while the other two were considered as “heat/cool” condition. All the MDSC tests were carried out under nitrogen (gas purge rate = 50 ml/hr).
32 (the gas purge rate is 50 ml/hr). The final SSA curves were normalized by both the weight fractions of PVOH.
To characterize the lamellar thickness measured by DSC, the Gibbs-Thomas equation was used: 0 0 2 ( ) m
f m m
T l
H T T
=
− (12)
where Tm,
0
m
T , ,Hf and l represented the observed melting temperature, the equilibrium
melting temperature, the surface free energy per unit area of PVOH chain folds, the heat of fusion per unit volume of the crystals, and lamellar thickness, respectively. Herein, the
f
H
and
0
m
T
33 3. RESULTS AND DISCUSSION
3.1. Freeze-dried PVOH gels 3.1.1. Appearance of gels
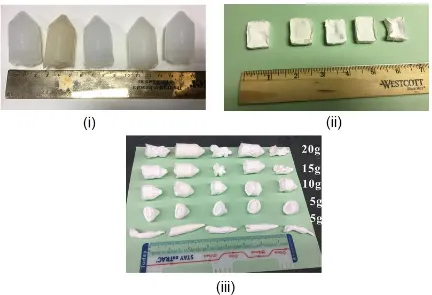
Figure 3.1. Physical appearance of hydrogels and foams (from left to right in digital images: P-H (F), SPP-H (F), CD-H (F), G-H (F) and U-H (F)): Appearance of hydrogels (i), rectangular foams after drying process (ii), and cone-shape foams after freeze-drying process (iii).
34 Addition of -CD and urea even caused severe shrinkage and failure of foam formation. With respect to gelatin and SP, the former helped to a form a foam with no shrinkage, but a relatively coarse surface, while the latter helped to form a perfect foam. This effect on foam formation was more obvious when the weight and length of the foam increased. In addition, it was also found that the appearance of foams depended upon the physical shape of the forms. When the hydrogels were of rectangular shape, the foamability of -CD and urea systems (refer Figure 3.1 (ii)) was not as much influenced as exhibited when in the form of cone-shape. It should be noted that, in PVOH/SPP and PVOH/gelatin hydrogels systems, phase-separation occurred where the SPP or gelatin phase was dispersed within the PVOH continuous phase. The role of phase separation in solid foam formation will be discussed in a later section.
3.1.2. Water content and swelling ratio
Figure 3.2. The water content (i) and swelling ratio (ii) of hydrogels and foams.
35 foams with good appearance (SPF and G-F) showed higher swelling ratios. The SR value of P-F was in the middle, while the other systems exhibited low SR values. These results correlated well with the appearance of the foam because the network structure of the shrunken foams was severely damaged and thereby compromised water uptake.
3.1.3. Porosity
Figure 3.3. Porosity of different systems calculated using solvent uptake method. To further verify this viewpoint, the porosity results are shown in Figure 3.3. One can see in Figure 3.3 that foams with better appearance/foamability (SPP-F and G-F) exhibited higher porosity. This difference was more obvious with respect to the samples with cone shape (e.g., the porosity of U-F with cone-shape was extremely low). In general, the porosity results correlated well with the foams’ physical appearance and swelling ratio results.
3.1.4. TGA tests
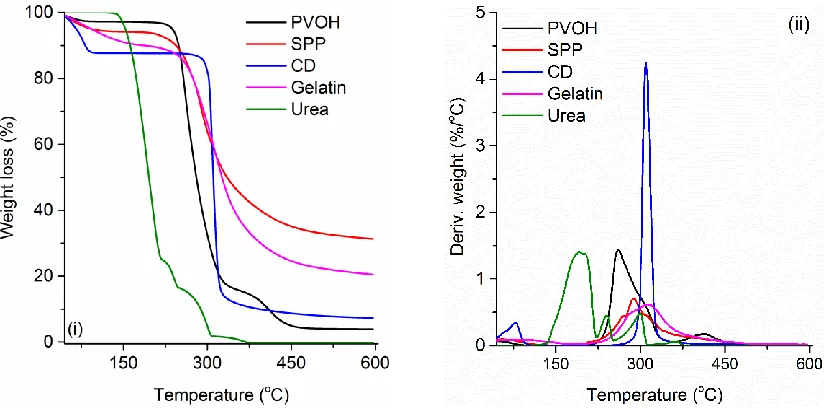
36 natural components in the thermal stability, TGA and DTG curves of single component systems in powder form (not the composite foam) are shown in Figure 3.4. Briefly, all the components exhibited an initial degradation step for the moisture removal followed by 1 to 3 further degradation steps, which differed in systems and could be assigned to elimination of -OH groups (specifically for PVOH), oxidation, chain scissions (for PVOH, SP and gelatin) and carbon chain rearrangements [106-109].
37 Figure 3.5. Thermal stability of different foams: TGA curves (i), DTG curves (ii) and thermal decomposition temperature at 10 wt% (T10) and 50 wt% (T50) weight loss (iii).
38 Figure 3.5 (ii) shows the DTG curves of different samples. For P-F, there are three degradation steps, which were assigned to the water/moisture loss from the never-dried sample (a small rounded DTG peak up to ~ 130 oC), the water elimination of PVOH molecules (a sharp DTG peak up to ~ 390 oC), and the carbonaceous materials that were formed during the pyrolysis process (a medium DTG peak up to ~ 540 oC). In comparison with P-F, the 2nd DTG peak intensity of CD-F and U-CD-F increased, indicating that CD and urea accelerate the elimination of water from PVOH polymeric chains. However, for the samples of SPP-F and G-F, the 2nd DTG peak was greatly suppressed and new DTG peaks located at 330 oC and 352 oC were observed. The existence of the new DTG peaks were unexpected because even the DTG peak temperature of pure SPP and gelatin were found at 288 and 313 oC, which were lower than those of the new DTG peak. Further characterizations (i.e., TGA coupled with FTIR) should be considered to explain this phenomenon. In addition, the peak intensity of this new peak was lower than that of the 2nd DTG peak (the degradation step of eliminating -OH groups of pure PVOH), which also revealed the stabilizing effect of SPP and gelatin.
To better characterize the thermal stability, the values of T10 and T50 are shown in Figure 3.5 (iii). One can see that the T10 and T50 values of CD-F and U-F were close to those of pure PVOH. However, SPP-F and G-F exhibited an increase of 9.9 oC and 16.4 oC for T10, and 43.2 oC and 47.9 oC for T50, respectively. These results indicated that soy protein polymer and gelatin can considerably increase the thermal stability of PVOH.
3.1.5. FTIR tests
39 of the 2nd component, SPP-F, G-F, CD-F, and U-F exhibited new characteristic peaks at 1641, 1646, 1638, and 1660 cm-1, respectively, which corresponded to the carbonyl groups in amide component of SPP and gelation, the OH groups in the glucose moieties of CD [110], and the carbonyl group in the urea.
Figure 3.6. FTIR of different materials: (i) Different pristine powders: (1) SPP; (2)
-CD; (3) gelatin; (4) urea; (ii) Different foams: (5) P-F; (6) SPP-F; (7) CD-F; (8) G-F and (9) U-F.
40 frequency region (from 1677 to 1660 cm-1), verifying that urea did participate in the hydrogen bonding.
With respect to SPP-F and G-F, the -OH stretching bands were at 3271 cm-1 and 3277 cm-1, respectively, which shifted to higher frequency. Such shifts possibly indicated that the hydrogen bonding of PVOH molecules was dissociated by the soy polymer and gelatin chains [111], even though PVOH/SPP and PVOH/gelatin systems were immiscible and exhibited phase separation behavior. If so, this was reasonably ascribed to sites such as amide groups to interact with PVOH molecules and therefore disturb H-bonding between PVOH molecules. However, compared with the afore-mentioned CD and urea systems, the shift of -OH groups to the higher frequency region clearly indicated that the new hydrogen bonding between PVOH and SP/gelatin was not formed. These were also supported by the fact that no shift of the carbonyl groups in the amide groups of the binary system to the lower frequency region was observed. In other words, the interaction between the PVOH and SPP/gelatin existed, but could not be defined as hydrogen bonding; instead, it was possible that it helped to deconstruct a portion of the hydrogen bonding character in PVOH.
3.1.6. DSC studies
41 interesting to find that the U-F system did not show a well-defined endothermic peak at ~ 150 oC but exhibited a small bump ((5) in Figure 3.7 (ii)), which made the mathematical interpretation (integration) very difficult. Moreover, U-F exhibited a very broad melting peak, whose enthalpy was calculated to be 139.1 J/g, which was too high to be the endothermic peak of PVOH crystals. Pure urea exhibited a very sharp endothermic peak at 137.6 oC (not shown) corresponding to the melt of urea crystals [113]. Therefore, such an interesting phenomenon should not be simply attributed to the “rule of mixtures” caused by urea, but instead might be attributed to general heat energy that disrupts interactions.
Figure 3.8 showed the curves of the 2nd heating scan and the cooling scan. As shown in Figure 3.8 (i), the endothermic peak at around 150 oC observed in Figure 3.7 (ii) disappeared, which was due to the irreversible nature of these interactions and thermal history. In addition, the melting peak during the 2nd heating scan seems to be sharper than those in the 1st heating scan. With respect to the cooling scan (Figure 3.8 (iii)), only one exothermic peak corresponding to the crystallization of PVOH was observed.
42 between PVOH and urea; therefore, this value is missing in Figure 3.9 (i) and the melting peak temperature value was only of reference value (technically, it is not a melting peak).
43 Figure 3.8. The 2nd conventional DSC heating curves (i-ii) and crystallization curves (iii) of different foams: (1) P-F; (2) SPP-F; (3) CD-F; (4) G-F and (5) U-F; (ii) is the enlarged view of (i).
44 system), there were several differences. The melt enthalpy slightly increased for SPP-F (by 1.4 J/g) and CD-F (by 5.7 J/g), while a large increase was observed for P-F (by 15.3 J/g) and G-F (17.5 J/g), respectively. Regarding the melting peak temperature, SPP-F exhibited a decrease of 3.9 oC, while the rest did not change much. It was deduced that the first heating scan disrupted the interaction between the 2nd component and PVOH which might be weakened during the 2nd heating scan. As a result, there may be more freedom for PVOH to crystallize and give rise to higher melt enthalpy. This assumption was further evidenced by the difference between the onset and end of the melting peak that characterizes crystal distribution. As shown in Figure 3.9 (v), the value of the 2nd heating scan is much lower than the corresponded value in the 1st heating scan. It should be noted that that the increase in the melt enthalpy of G-F system was even higher than the increase in P-F; in sharp contrast, SPP showed much less increase in melt enthalpy. Further research needs to be considered to explain this interesting difference.
46 Figure 3.10. SSA curves of different foams.
47 crystals for further analysis. With respect to other systems, CD-F exhibited the same number of Ts, while G-F, U-F and SPP-F showed four, one and zero Ts, respectively.
(i)
(ii) (iii)
(iv) (v)
48 Figure 3.12. The parameters derived from SSA curves: (i) relationship between Tm and Ti; (ii) melt enthalpy of different “fingers” versus temperature; (iii) melt enthalpy fractions versus lamellar thickness; (iv) melt enthalpy derived from SSA peaks and the whole curve (the blunt endothermic peak before different thermal fractionations was also included).
49 were still in the liquid/molten state (the crystals were melted) and therefore the influence of self-nucleating and annealing introduced by isothermal treatment can be ignored.
50 enthalpy. The melt enthalpy value of SSA melting peaks and the whole endothermic peaks are shown in Figure 3.12 (iv). The enthalpy of the whole endothermic peaks (total) is higher than that of SSA peak(s). This again verified that under the condition of current SSA protocol some chains that are less crystallizable still existed and their crystals melted at lower temperature below the pre-designed SSA thermal fraction region.
The ice-water transition of different hydrogels was studied by DSC (Figure 3.13 (i)). The melt enthalpy of different ice crystals was calculated, and the results are shown in Figure 3.13 (ii). The melt enthalpy value of SPP-H was lower than the others, and the melt enthalpy values of P-H, G-H, CD-H and U-H were very close to each other. It should be noted that the enthalpy values obtained herein are higher compared with previous work [118], and we did not calculate bonded water and free water reported elsewhere [112, 118]. It was quite possibly owing to the different crosslinking systems (the current study was not based on a chemical crosslinking effect). In addition, the enthalpy value was very sensitive to the selection of onset and end points for integration, which might also contribute to this phenomenon.
51 Modulated DSC was also used to test different systems. Compared with linear heating ramp of traditional DSC protocol, additional sinusoidal temperature oscillations were introduced, which can split heat capacity-related parameters (i.e., melting and glass transition) and temperature dependent parameters (i.e., crystallization and relaxation) that can be represented in the form of reversible and non-reversible heat flows, respectively [119]. In an ideal MDSC protocol (using a suitable combination of linear ramp, sinusoidal amplitude, and modulation period), the contribution made by both the reverse and non-reverse signals can be distinguished and therefore more accurately estimate different parameters (i.e., glass transition temperature, crystallinity, and the enthalpy of chemical reactions) [119, 120].
MDSC was used to test the hydrogels (wet state). For physically crosslinked hydrogels, the crosslinking effect that supported the gelation can be irreversibly broken into sols under heat, termed as the “gel-sol” process [10]. Therefore, it was postulated that DSC/MDSC was a very straightforward method to track “gel-sol”, and the measured enthalpy proportionally reveal the crosslinking strength. However, we found that traditional DSC was unable to characterize this process as shown in Figure 3.14 (iv), where unresolvable signal-noise was observed. This was mainly due to the evaporation of water especially close to the boiling point of water. The evaporated H2O (g) hit the DSC pan and lid and thereby created a “dancing” signal noise.
52 any unsuccessful modulation may tell another story. Nevertheless, based on the current study, the order of the enthalpy value for “gel-sol” was G-H > SPP-H > P-H > CD-H > U-H. The higher values of G-H and SPP-H was possibly attributed to the denaturation of SP and gelatin especially when considering the signal of “gel-sol” signal is so weak. The relatively low value of U-H suggested the weakest crosslinking effect, consistent with the lowest melting enthalpy and melting peak temperature observed in DSC results of different foams (refer Figure 3.9). In addition, the enthalpy value calculated in Figure 3.14 (i) was slightly higher than that of the corresponding one in Figure 3.14 (ii). This was because the total heat flow was the sum of reverse heat flow and non-reverse heat flow. In other words, the non-reverse heat flow also contributed to the enthalpy value of the total heat flow curves even though it did not show any endothermic peaks for the “gel-sol” process.
53 Figure 3.14. MDSC/DSC curves of different hydrogels: (i) Total heat flow MDSC curves; (ii) non-reverse MDSC heat flow curves; (iii) reverse MDSC heat flow curves; (iv) DSC curves of P-H.
57 Figure 3.17. The MDSC curves under heat/cool condition (3 oC/min): (i) P-F, (ii) SPP-F; (iii) CD-F; (iv) G-F; (v) U-F; the solid, dash and dot curves represents the total heat flow, the reverse heat flow and the non-reverse heat flow curves, respectively.
59 show two endothermic peaks but are of a single broad endothermic peak instead. This again verified that addition of urea induced the irreversible (non-reversible) interactions and was consistent with the aforementioned traditional DSC results. In addition, the existence of the blunt reversing melt peak in U-F also revealed that some crystals were still available even though the overall crystal growth was hindered.
When it comes to the heat/cool conditions, the state/shape of weak reversing heat flow, sharp non-reversing heat flow and the total heat flow were quite similar in most systems except U-F systems. As shown in Figure 3.16 (v), a dual endothermic peak in the total heat flow curves and non-reversing melt curves were observed; while no melting peak in the reversing melt curves was observed in Figure 3.17 (v). All these indicated more sensitivity of the urea system on the higher modulated temperature amplitude.
60 Figure 3.18. The reversing, non-reversing, and the total melt enthalpy derived from MDSC curves: (i) Heat-only condition (2 oC/min); (ii) heat/cool condition (2 oC/min); (iii) heat/cool condition (3 oC/min).
61 shown in Figure 3.18 (i)-(ii). All these trends were like the enthalpy values observed in the traditional DSC results (refer Figure 3.9). With respect to the dependence of modulated condition, the transition from heat-only condition to heat/cool slightly increased the non-reversing and total melt enthalpy, while the influence in the reversing melt enthalpy became less. The former might be attributed to more “ordering” caused by the instantaneous cooling. The latter might suggest that the crystals that contributed to the reversing melt peak were less disturbed by the modulated thermal conditions.
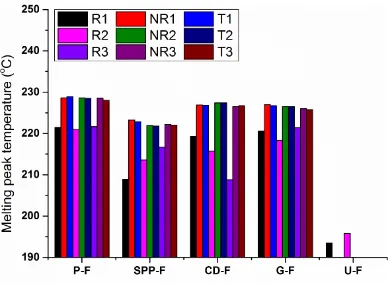
Figure 3.19. The melting peak temperature of different MDSC curves; the R, NR, T in the inserted legend represented reversing, non-reversing and the total heat flow curves, respectively; 1, 2, and 3 corresponded to heat-only condition (2 oC/min), heat/cool condition (2 oC/min), and heat/cool condition (3 oC/min), respectively.
62 the increasing and decreasing trend in the peak temperature of reversing melt transition was observed in S-F system and CD-F system, respectively, when heat-only was changed to heat/cool. U-F exhibited the lowest reversing melt peak temperature among systems, verifying the negative role exerted by urea on the crystal growth.
3.1.7. WAXS studies
Figure 3.20. Wide angle X-ray scattering (WAXS) curves of powder (i) a-pure PVOH powder, b-SPP powder, and c-gelatin powder, and foams (ii) a-P-F; b-SPP-F; c-CD-F; d-G-F and e-U-F.
![Figure 1.1. Typical examples of physically and chemically crosslinked hydrogels [3].](https://thumb-us.123doks.com/thumbv2/123dok_us/1729821.1220874/18.612.95.514.86.661/figure-typical-examples-physically-chemically-crosslinked-hydrogels.webp)
![Figure 1.2. Foaming window based on polymer strength and gas/melt equilibrium constant [11]](https://thumb-us.123doks.com/thumbv2/123dok_us/1729821.1220874/23.612.109.498.71.394/figure-foaming-window-based-polymer-strength-equilibrium-constant.webp)
![Figure 1.3. A schematic classification of gel applications [16].](https://thumb-us.123doks.com/thumbv2/123dok_us/1729821.1220874/24.612.99.521.73.459/figure-schematic-classification-gel-applications.webp)