JOURNAL OFVIROLOGY, Sept.1980,p.644-652 Vol. 35,No. 3 0022-538X/80/09-0644/09$02.00/0
Shared
Antigenic
Determinants Between Two Distinct Classes
of Proteins in Cells
Infected
with Herpes
Simplex
Virus
MARTIN
ZWEIG,`*
CONRAD J. HEILMAN,JR.,' HARVEYRABIN,2ANDBERGE HAMPAR3Carcinogenesis IntramuralProgram,' BiologicalCarcinogenesis Program,2 and Laboratory of Molecular Virology,NationalCancer Institute,3 Frederick Cancer ResearchCenter, Frederick, Maryland21701
Guineapig antisera and mousemonoclonal antibodiesagainst a
40,000-molec-ular-weight nucleocapsid protein (p40)
ofherpes simplex
virus types 1 and 2immunoprecipitated
40,000- and80,000-molecular-weight
classes ofsoluble pro-teins from infected cell extracts. The soluble40,000-molecular-weight
proteinclass
(intracellular p40) appeared
asacluster of threetofourclosely spacedbandsofproteins having molecular weights ranging between 39,000 and 45,000,whereas
thesoluble
80,000-molecular-weight
protein class(intracellular p80) appearedasadoublet of bands. The peptide map of intracellular p40 closely resembled the
maps of the p40 andp45 proteins of nucleocapsids, but it showed bothdifferences
and similarities when compared with thepeptide mapofintracellularp80.
Pulse-chase
experiments
suggested that intracellular p80 was not a precursor ofintra-cellular p40. We conclude that
the intracellular p40 and p80protein
classesshare common antigenic determinants, presumably reflecting similar amino acid se-quences, although they have distinct differences inproteinstructure.In our
laboratory
we have beenengaged
instudying
thenucleocapsid proteins
ofherpes
simplex
virus type 1(HSV-1)
andtype 2(HSV-2).
We have shown by competition immunoas-says that a40,000-molecular-weight
polypeptide, p40, which is amajor
component of thesenu-cleocapsids,
possessesboth
type-specific and
cross-reactive
antigenic
determinants(7).
Ra-dioimmunoprecipitation
studies withmonospe-cific antisera and
monoclonal antibodies haveindicated
thatp40
shares at least some of thesedeterminants
withanothernucleocapsid
protein,
protein
p45(14).
The
finding that nucleocapsid proteins p40
and
p45share
antigenic determinants led
us toexamine infected
cells for the
presenceof
other
proteins
whichmight
have the same determi-nants.Such
proteins
might be
precursors,poly-proteins,
orother
typesof derivatives which
could indicate
important relationships
among theseproteins.
Wefound that
antibodies against
nucleocap-sidp40 immunoprecipitated
40,000- and80,000-molecular-weight classes of
proteins
from in-fected cell extracts.The
40,000-molecular-weight class
(intracellular p40) appeared
to in-clude thenucleocapsid
proteins p40and
p45, whereas the80,000-molecular-weight
class (in-tracellularp80)
was notfound innucleocapsid
preparations. Pulse-chase
experiments
failed to show aprecursor-product relationship
betweenintracellular
p80
andintracellularp40.
The pep-tidemaps of these two classes ofproteins
showed bothdifferences
andsimilarities.MATERIALS AND METHODS
Cells, viruses,andantibody preparation. HSV-1 strain MAL andHSV-2 strain MS were grown in Vero cells as previously described (7). Guinea pig antisera against HSV-1 and HSV-2 p40's were
pre-pared byimmunizing animals with purifiedp40protein obtained frompolyacrylamide gels (7). Ascites fluids containing monoclonal antibodies against p40 were produced by intraperitoneal inoculations of hybridcell
lines 1D4 (whichsecretesanti-HSV-1 antibodies) and 3E1 (whichsecretesanti-HSV-2 antibodies) (14) into
BALB/cmiceprimed with Pristane (Aldrich Chemical Co., Milwaukee, Wis.),asdescribedby Lostrum et al.
(9).
Purificationof virions. Virions were purified
es-sentially as described by Spear and Roizman (13).
Cells infected withHSV-1 orHSV-2were harvested 24hpostinfection, suspended in2volumes of1 mM dibasic sodiumphosphate (pH8.2)containing0.1 mM
phenylmethylsulfonyl fluoride, and allowed to swell
for10minat0°C.Thecellsweredisrupted by dounce homogenization,andthe nuclei werepelletedby cen-trifugationat800xgfor10min andstoredat-70°C until used for the purification of nucleocapsids (see below). Debris was removed from the cytoplasmic fraction bycentrifugation in aSorvall SS34rotor at 8,000 rpm for5min. The virions in thecytoplasmwere sedimentedby centrifugation at 60,000 x gfor 1 h, suspendedin 1 mM dibasic sodium phosphate, and subjectedtosonic treatment. After incubationinthe presenceofDNase (50,ug/ml)andRNase (50
tLg/ml)
for30min at room temperature, the virionsamples werelayeredonto34-ml5to 30%(wt/vol) dextranT1O(Pharmacia Fine Chemicals, Inc., Piscataway, N. J.) gradients in 1 mM dibasic sodium phosphate and centrifugedfor 1 h at20,000 rpmin aBeckmanSW27 rotor. The virion-containing band wascollected, di-644
on November 10, 2019 by guest
http://jvi.asm.org/
lutedapproximatelyfourfold with 10 mM Tris-hydro-chloride (pH 7.6)-i mM EDTA (TE buffer), and pel-leted by centrifugation at 25,000 rpm for 1 h in a Beckman SW27 rotor. The virionpellet was suspended in asmall volume of TE buffer and was layered onto an11-ml10to50%(wt/wt)potassiumtartrategradient in TEbuffer. Centrifugation of the gradient was per-formed with aBeckman SW41 rotor for 2 h at 25,000 rpm. The virusband was collected, dialyzed against TEbuffer, and storedat-70°C.
Purification of nucleocapsids. Nuclei of infected cellsobtained asdescribed above were suspended in about 2volumes of 0.1 M Tris-hydrochloride (pH
8.0)-1.5mMMgCl2-0.1mMphenylmethylsulfonyl fluoride. The nuclei were lysed by adding sodium deoxycholate to a final concentration of0.5%, followed by sonic treatment.The nuclear lysates were incubated at room temperature for30 min inthe presence of50,igof DNase perml;thiswasfollowed by centrifugation at 8,000 rpmfor5mininaSorvall SS34 rotor to remove debris.Nucleocapsidswerepurifiedfromclarified nu-clear lysatesbycentrifugation through 35% (wt/vol) sucrose,followedby centrifugationina10to40% (wt/ wt) sucrosegradient, as describedpreviously (6, 7, 13).
Radiolabeling ofcells. Cells were washed once with methionine-freeEagleminimalessential medium containing 5%dialyzedheat-inactivatedfetal calf se-rumand thenlabeled with 100
,uCi
of[3S]methionine(800 to 1,200 Ci/mmol; Amersham Corp., Arlington
Heights,Ill.)perml in thesamemethionine-free me-dium for 1 to 4h. The cell sheetwas thenwashed twice withice-cold Tris-buffered saline (pH 7.4) and
scraped, and the cells weresuspended in cold Tris-buffered saline. The cells were sedimented by
centrif-ugationat800xgfor 10 min, and the cellpelletswere storedat-70°Cuntilused.
Preparation ofcellextractsanddisrupted vi-rus particles for immunoprecipitation. Cell ex-tractswereprepared by suspending the
[3S]methio-nine-labeledcells in buffer A (0.1 M Tris-hydrochlo-ride
[pH
8.0], 10%[vol/vol]glycerol, 0.5% Nonidet P-40, 0.5%sodium deoxycholate,0.2mM phenylmeth-ylsulfonylfluoride)
andincubating them for1h at4°Cwithshaking.The extracts were clarified by
centrifu-gation at60,000xgfor1 h.[3S]methionine-labeled
virionsandnucleocapsidswere disrupted by heating at 100°C for 5min in 0.5% sodium dodecyl sulfate
(SDS)-2.5%
/i-mercaptoethanol-0.05
M Tris-hydro-chloride (pH8.0),followedby a10-fold dilution with buffer A (14).Immunoprecipitation and SDS-polyacryl-amide gel electrophoresis. Antibody (20
jl)
was incubated witha0.5-mlportion of eithercellextract ordisrupted virusparticles for 3 h at 4°Cand then further incubated for1hwith 0.12 ml ofa33%(vol/vol) suspensionofprotein A-Sepharose CL-4B beads
(PharmaciaFine Chemicals, Inc.). Afterincubation, the beads werewashed with 0.5 MLiCl-0.1 M Tris-hydrochloride (pH 8.0)-1% B-mercaptoethanol, sus-pendedin anequal volume of 2% SDS-20% glycerol-5%
,8-mercaptoethanol-0.125
M Tris-hydrochloride (pH 6.8)-0.004% bromophenol blue, and heated at 100°C for 5 min, as previously described (14). The proteinswerethenseparated by electrophoresis on a 5to20%polyacrylamide gel gradient containing SDS(7), and autoradiographs or fluorographs were pre-paredonKodakSB-5 X-rayfilm(1).Theabsorbances at 595 nmof the bands in theautoradiographs and
fluorographs weremeasured with a scanning densi-tometer (Transidyne General Corp., Ann Arbor, Mich.)
Peptide mapping. Proteins obtained from poly-acrylamide gelswerepartiallydigested with Staphy-lococcusaureusV8protease(Miles Laboratories, Inc.,
Elkhart, Ind.), and the peptides were analyzed by
SDS-polyacrylamide gelelectrophoresisessentiallyas described by Cleveland et al. (4) and Bordier and
Crettol-Jarvinen (2).Afterseparation by
electropho-resis,[3S]methionine-labeled proteinswerelocated in driedunfixedgels by alignmentwithautoradiographs,
and rectangular segments (0.5 by 1.5 cm) of lanes containing theseproteins were excised from the gel
slabs and incubated in 0.125 M Tris-hydrochloride (pH 6.8)-0.1%SDS-1 mM EDTA for 30 minatroom temperature.Thegelsectionsweredrained andplaced
in sample wells (width, 2 cm) of second-dimension
polyacrylamide gels,whichwerecomposedofa7-cm 5 to20%gradient gelbeneatha3-cm4%stackinggel.
The gelsegmentswere orientedsothat their bands wereparalleltothedirection ofelectrophoresisin the
second-dimension gel, and then they were covered withmelted(55°C)1% agarose in 0.125 M
Tris-hydro-chloride (pH 6.8)-0.1% SDS-1mMEDTA. S.aureus V8 protease in 0.125 MTris-hydrochloride (pH
6.8)-0.1% SDS-1 mM EDTA-10%glycerol-0.004%
bromo-phenolbluewasoverlaidonthehardened agarosegel,
andelectrophoresiswasconductedatlowvoltage(25 V) for 20 h to allow partial proteolytic digestion to
occur during the stacking phase of
electrophoresis.
Afterelectrophoresis,thegelswereprocessedfor
fluo-rographyonKodakSB-5 X-rayfilmbythe method of Bonner andLasky (1).
Competition
immunoassays.Purified virions andnucleocapsids were disrupted in 0.01 M
Tris-hydro-chloride (pH 7.8)-0.01 M NaCl-0.1% Triton X-100 containing 1% SDS and5mMdithiothreitolbyheating
at 100°Cfor5min.The preparationsweretestedat
serial twofold dilutions in the same buffer without
SDSordithiothreitolforabilitytocompetewith
'25I-labeled HSV-1 strain MALnucleocapsid p40 (10,000
cpm) forbindinglimitingconcentrations of
guinea
pig
antiserumagainstHSV-1
nucleocapsid
p40.Antiserum was used at a dilution of 1:400, which precipitated approximately 35% of thel"I-labeled
p40.Prepara-tionsof
'"I-labeled
p40,theantiserum,and thereac-tion condireac-tions have been described
previously (7).
RESULTS
Reaction of
guinea pig
antiserum
with
cell
extracts. HSV-2-infected anduninfected
cellslabeled
with[3S]methionine
weredis-rupted
withdetergents
and were clarifiedby
high-speed
centrifugation,
which
sedimented
vi-rusparticles and insoluble proteins.
Alarge
number
ofsoluble
virus-specified
proteins
wereobserved
ininfected
cell extractsby
SDS-poly-acrylamide
gel
electrophoresis
(Fig.
1,lane
A),
asevidencedby
their absence inuninfected
cellon November 10, 2019 by guest
http://jvi.asm.org/
646 ZWEIG ET AL.
A
B
C
D
E
F
G
J. VIROL.
-
p155
:_
_
_p50
*. ....:..
._SUhi - p40
0e-
p32
_
p12
FIG. 1. SDS-polyacrylamide gel electrophoresis of soluble cell extractproteins immunoprecipitated by guinea pigantiserumagainstHSV-2nucleocapsid p40. HSV-2- andmock-infected cells werelabeled with [35S]methioninebetween 18 and 22 hpostinfection. Extractswerepreparedandreactedwith eitherguinea
pig antiserum against HSV-2 nucleocapsid p40orcontrolguinea pig serum. Proteinsfrom cellextracts,
immunoprecipitates, and nucleocapsids were separated by electrophoresis, and an autoradiograph was prepared.LaneA, proteins ofHSV-2-infectedcellextracts;laneB,theproteins of mock-infectedcellextracts; lanes C andD,proteinsprecipitated from HSV-2-infectedcell extractsbyguineapigantiserumagainst HSV-2nucleocapsid p40 (lane C)andby control nonimmuneguinea pigserum(lane D); lanes E andF, proteins precipitated from mock-infectedcellextractsby guinea pigantiserumagainstHSV-2nucleocapsid p40 (lane E)andbycontrol nonimmuneguinea pigserum(lane F);laneG,proteins ofpurified
[3"SJmethionine-labeled
HSV-2 nucleocapsids. Themajornucleocapsidproteinsaredesignatedatthe right ofthe autoradiograph. Thep45 band is the minor bandimmediately beneath p50.
extracts (lane B). Guinea pig antiserum against the HSV-2 nucleocapsid p40 protein immuno-precipitated two classes of viral proteins from infected cellextracts (laneC); thesewere
intra-cellular p80, which appearedas aband doublet,
and intracellular p40, which appeared asthree orfour closelyspaced proteins encompassinga
molecular weight range between 39,000 and
45,000. This antiserum didnotprecipitate these protein classes from uninfected cell extracts
(lane E), and nonimmune guinea pigserum did
notprecipitate them from eitherinfected (lane D) oruninfected (lane F) cellextracts. Compa-rableresultswereobtained with HSV-1-infected
cellextractsand antiserum against HSV-1 p40
(see below). Labeled proteins were not immu-noprecipitated from infected cells incubated
with['4C]glucosamine, suggesting that intracel-lularp40 and p80werenotglycoproteins (data
notshown).
Synthesis of intracellular p40 and p80 afterinfection. Inanexperiment in whichcells were pulse-labeled with [35S]methionine at in-tervalsafterHSV-2infection, synthesis of intra-cellular p40 and p80 was first detected by
im-munoprecipitation with guinea pigantiserum at
about 3 h postinfection (Fig. 2). The rate of
production of these protein classes remained roughlyconstantbetween6 and 24 h postinfec-tion, but considerablylessintracellularp80was
usually precipitated at 24 h postinfection.
Al-though thecauseof thisreductioninprecipitable intracellular p80 is uncertain, substantial cyto-pathic effects wereevidentat this latestage in
infection, and intracellularp80 may have been
selectivelylostinto themediumordegraded by
proteases.Antiserumwasreactedwith extracts
of infectedcells whichwerepulse-labeledfor15
min and then chased forupto4h with isotope-free medium (Fig. 3).
SDS-polyacrylamide
gel electrophoresis showed that theratio of thein-tensities of the bands of intracellular p40 and
p80 remainedconstantduringthe chaseperiod, suggestingthat intracellularp40andp80didnot
serve asprecursorstooneanother.
Reaction of guinea pig antiserum with purified proteins. To verify that intracellular p40 and p80 possess common antigenic
deter-minants, each protein class was purified from
polyacrylamide gels and was successfully
im-munoprecipitated by guinea pig antiserum
against nucleocapsid p40 (Fig. 4). Therefore, it
Ji'
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.510.88.403.72.295.2]SHARED ANTIGENIC DETERMINANTS OF HSV PROTEINS 647
Hours Post-infection
1
2
3
4
5
6
7
8
-
p80
-
p40
9
10
11
12
16
24
- _I
-p80
[image:4.510.57.249.79.323.2]. ~~ ~ ...
FIG. 2. Synthesisofintracellular p40 andp80after
HSV-2infection.Cellswerelabeled with
[35S]methi-oninefor30min,terminatingattheindicated times
after
infection.
Cell extracts wereprepared
andre-acted withguinea pigantiserumagainstHSV-2
nu-cleocapsid p40. Theproteinsinthe
immunoprecipi-tates wereseparatedby
SDS-polyacrylamide
gel
elec-trophoresis,andanautoradiographwasmade.
isunlikely that the precipitation of intracellular p80 from infectedcellextractswasduetospecific
ornonspecific bindingtointracellular p40. Fur-thernore, although the p40 protein exists in disulfide-linkedcomplexes in nucleocapsids (13), soluble disulfide-linkedcomplexes containing in-tracellularp40orp80werenotdetectedby
non-reducingSDS-polyacrylamide gel electrophore-sis(datanotshown).
Reaction of
monoclonal
antibodies with
infected
cell extracts.Recently,
weestab-lishedmousehybridcelllines whichsynthesize
monoclonalantibodiesagainst the p40 and p45 proteins of HSV-1 and HSV-2 nucleocapsids (14). Ascites fluids containing high titers of
mon-oclonal antibodieswere prepared andwere
re-acted withextractsofcells infected with either HSV-1 or HSV-2. The anti-HSV-1 p40
mono-clonal antibody produced by cell line 1D4
pre-cipitated intracellular p40 and p80 from only HSV-1-infected cell extracts (Fig. 5A). In
con-trast, the anti-HSV-2 p40monoclonal antibody produced by cell line 3E1 precipitated both
HSV-1 and HSV-2 intracellular p40 and p80
(Fig. 5B), although the homologous proteins
were
precipitated
athigher
dilutions of
3E1an-tibody
than
werethe
heterologous proteins.
Guinea
pig
antisera
against
the
nucleocapsid p40
proteins of
HSV-1 and HSV-2 also
precipitated
homologous
proteins
athigher
antibody
dilu-tions, but the
differences in the dilutions which
precipitated the
homologous
and
heterologous
proteins
were not asgreat asthe
difference
ob-served with the
3E1monoclonal
antibody (Fig.
5A and
B).
Peptide
mapsof
immunoprecipitated
proteins. The
finding
that
monospecific
anti-seraand
monoclonal antibodies reacted with
HSV
polypeptides
having
different
molecular
weights
indicated
that these
n~~~
polypeptides
pos-a) U) u) cu
u) Xu cu s~
Cu . z ._
U U
C C 0
.T
EEg
Eo o
vr
o
7C)
CD N'4p80-p40-
p4O
...~ ~
_ :..FIG. 3. Autoradiogram of a polyacrylamide
gel
showingtheimmunoprecipitationof
intracellularp40andp80 fromcellspulse-labeledwith
[35S]methionine
and chased in nonradioactive medium. At16h
after
HSV-2infection, cellswereincubatedfor15min in
methionine-free medium. The medium wasthen
re-moved, and the cellswere
pulse-labeled
for
15minwith
[35S]methionine
infresh
methionine-free
me-dium. After the cellswere washed three times and incubated (chased) in complete nonradioactive me-diumfortheindicatedperiodsof time, cellextracts werepreparedand incubated with
guinea pig
anti-serumagainst HSV-2nucleocapsid
p40.
Theimmu-noprecipitatedproteinswereseparated by
SDS-poly-acrylamide gelelectrophoresis,and
autoradiographs
wereprepared. The relative intensities
of
thebands weremeasuredonscanningdensitometertracings
of
autoradiographs
preparedaftervarying intervalsof
exposure. VOL. 35,
1.980
on November 10, 2019 by guest
http://jvi.asm.org/
[image:4.510.289.433.236.488.2]648
ZWEIG ET AL.
A B C
p80
_i
p40I
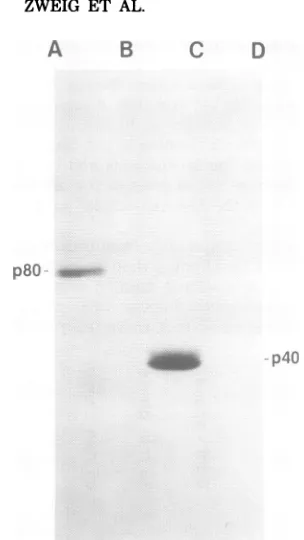
FIG. 4. Immunoprecipitation of purified intracel-lularp40andp80byguineapigantiserum against HSV-2 nucleocapsid p40. An extract of HSV-2-in-fectedcellslabeled with[35S]methionine between18 and22 hpostinfectionwasreactedwithguineapig antiserum againstHSV-2nucleocapsid p40,and the immunoprecipitatedproteinswereseparated by
SDS-polyacrylamide gel electrophoresis.Intracellularp40 andp80werelocated in thegelby alignmentwithan
autoradiograph, andgelsegmentscontaining intra-cellularp40andp80werecut out. Theproteinswere
eluted into bufferA and reacted witheitherguinea pigantiserumagainst p40orcontrolguineapig se-rum. The proteins in the immunoprecipitates were
separated bySDS-polyacrylamide gel electrophore-sis, andafluorographwasprepared. Proteinswere
precipitated by reacting purified intracellular p80 with antiserum against p40 (lane A) and control
serum(lane B)andbyreacting purifiedintracellular
p40withantiserumagainst p40 (lane C)and control
serum(lane D).
sess commonamino acidsequences. We tested
this hypothesis by an analysis of the peptide
mapsof
immunoprecipitated
proteinsaftersep-aration by
SDS-polyacrylamide
gel electropho-resis, partial digestion with S. aureus V8 pro-tease,and resolutionof thepeptides byelectro-phoresis inasecond
SDS-polyacrylamide
gel (2,4). The peptide maps of the intracellular p40
andp80 classesrepresent thesumsoftheprotein bandscomprisingeachclass,since thevery
sim-ilarmobilities of thebandspreventedtheir clear
resolution andseparationinthefirst
electropho-resis
step.
Thepeptide map
ofintracellular
p40
obtained
afterdigestion
with 1,ug
ofV8protease
contained seven oreight peptides,
which hadmolecular
weights
ranging
from40,000
(intact
intracellular p40)
to8,000 (Fig. 6).
Nucleocapsid
p40
andp45
had the samepeptide maps, except
thatthey
lacked
the8,000-
and11,000-molecu-lar-weight
peptides.
Thenucleocapsid p45
digest
wasalso
deficient
in a14,000-molecular-weight
peptide
whichwaspresent
in thedigests
ofnu-cleocapsid
andintracellular p40's. The digest
ofintracellular
p80 contained
intactintracellular
p80
andonly
three smallerpeptides,
whichco-migrated
withpeptides found
in theintracellular
p40
digest.
It is uncertain whether this comigra-tion is areflection
ofthe
presenceof identical
peptides
inthese
digests.
On the otherhand,
thedigests
ofintracellular and
nucleocapsid p40's
included peptides which did
notcomigrate
withany
oftheintracellular p80 peptides. The
pep-tides
continued to show these common and dis-tinctmigration properties
when theprotein
classes weredigested
withdifferent
amounts of S. aureus V8protease (Fig.
7).
Reaction
ofantibodies with virion
pro-teins.Virions
ofHSV-1 and HSV-2
were puri-fiedfrom
cytoplasmic extracts by
centrifugation
in
dextran T10
andpotassium tartrate
gradients.
Electron
microscope observations indicated that
theHSV-1
virionpreparations contained
about 10 to 15%unenveloped
nucleocapsids, whereas
theHSV-2
preparations contained
as many as 50%unenveloped particles. These
findings
arereasonably consistent with
those obtained
by
Cassai
et al.(3). Because of their
higher purity,
weconcentrated
ourefforts
onanalyzing
prepa-rations ofHSV-1 virions
todetermine whether
they
possesspolypeptides
immunologically
re-lated
top40. We
were notable
toidentify
di-rectly
nucleocapsid p40 and
p45 invirions
withcertainty
by electrophoretic
analysis because
of thepresence
ofproteins
having
amigration
sim-ilar
tothat of
nucleocapsid
p40
(Fig.
8). Guinea
pig antiserum and
mousemonoclonal antibody
against HSV-1
nucleocapsid
p40
precipitated
much smaller amounts ofnucleocapsid
p40and p45 from virion preparations thanfrom
prepa-rationsof
nucleocapsids, whereas
aproteinhav-ing the
mobility of intracellular p80
was notprecipitated from
either virion ornucleocapsid
preparations. We were notable
tocompareac-curately
the amounts of p40 and p45 presentin
virionswith the
amountsfound
innucleocapsids
becausethe
preparations
may have haddiffering
amounts
of
contaminating
extraneousproteins.
DISCUSSION
Through
the use of monoclonal antibodiesandJ. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:5.510.83.235.60.330.2]co
V7zOL
CL
m
V~~~19
.: ts >*-9=
5cn
)9L
co L
I
tZ0
L
w
0 =
-W
4
9SZ
b9
9L
b
L
'a)
-W0~
C.)
L-x
s
-.E u
-4-(A ._
ofo
CL
' 0.0g C, > =
,O
Dn
0C .) I
C
Cu50X
IC 0
OD: *5) .5
r4:<
o
SL QL
10. _. It .C 0
17zO
L
9SZ
179
9L
L
a'i
.I.
17ZO L
99Z
b9
9L
L
I
.i1
co 0L
0o
'0
I._~0aLO4
)
)c1
0x 0)
CO'0.-a-- cu@410
a._ U,
a)
.0
C
c
4
-0
0 (1)
0
Cu
I
L-:01
-N Q6
.~
bqs, t J
_t
p tCf
C)
o*
cZ
0
bo
t-4
!
.!.
L.2-.s.
L.s
o .
.s
.gee.
O .50 u
U)
aL)
0 .0
I
c
7
-0
0
0
*1Z
I
u
t.
J
I
,,I.;---.
I..
%-.R %.j
co RT
CL CL
on November 10, 2019 by guest
http://jvi.asm.org/
650 ZWEIG ET AL.
A
INTRACELLULAR
p80 p40
I
0K :
80K-
I
28K -_
17K-
W_
1 1
K-p80 p40
[image:7.510.61.260.62.553.2]INTRACELLULAR
FIG. 6. Peptide mapsof
guinea pig antiserum agai
p40.[35S]methionine-labele tractsandHSV-2-disruptec acted withguineapiganti tated proteins were separ amidegel electrophoresis,g
proteinswereexcisedand p secondpolyacrylamidegels
fixedintoplacewithmolter laidwith S.aureusV8protec
in theseconddimensionwas
B
monospecific antisera,
wehave shown that HSV-NUCLEOCAPSID 1and HSV-2 induce thesynthesis of twomolec-ular weight
classes of soluble intracellularpro-p45
teins; these are intracellular p40 and p80, which |p40 areimmunologically
related to each other and topolypeptides p40 and
p45,which
areassoci-ated with
nucleocapsids isolated from cell nuclei.
Proteins
having the
mobility of intracellular p80
werefound
inneither virions
nornucleocapsids.
The
finding of
anucleocapsid protein
sharing
antigenic determinants with
anon-nucleocapsid
protein
wasalso observed in
cellsinfected with
simian
herpesvirus
SA8
(data
notshown),
indi-cating that this
propertyis
notrestricted
toHSV. The
immunological data
suggestthat
HSV
intracellular
p40 and p80
possess commonamino
acid
sequences,although
the
peptide
mapsof
these
protein classes showed differences in
atleast
someof their
methionine-containing
pep-tides. The number and size of these peptides
aredependent
onthe number and distribution of
methionine residues and accessible
proteolytic
cleavage
sites,
aswell as onthe molecular
weight
of the
protein.
Therefore, it is
notinconsistent
that
the
peptide
mapof
intracellular p80
con-tains
fewer
methionine-containing peptides
than
the
peptide
mapof the
smallerintracellular p40
class. The data indicate that regions of
intracel--40K -- *
lular
p40
and
p80
differ in primary structure,
whereas other
regions
possessrelated amino acid
sequences.Large and
smallT
antigens of simian
- 28K _*virus
40 and polyoma virus contain unique and
-25K
common amino acid sequences (8, 10, 11) which
- 18K are generated by a mechanism involving
post-- 17K - _ transcriptional splicing of messenger RNA.
Elu-cidation of the mechanism
responsible for the
-14K - _
putative common amino
acid sequences and of
-11K
|distinct
peptide maps of
intracellular p40
and
p80 would require characterization of the
struc-- 8K
tural genes and the mRNA specifying these
pro-tein classes and studies
ontheir
primary
struc-ture.
The
causesfor the
differences in
mobility of
P15
nucleocapsid p40
and
p45
and the
closely spaced
P4
^0
bands of
intracellular
p40
and
p80
areprobably
related
tostructural
variationsamong the poly-NU CL EOCA PSI Dpeptides
in these bands. These variations
maybe
due
topost-translational modifications,
suchproteins precipitated
by asphosphorylation or acetylation, to proteolyticnst
HSV-2nuckocapsid
cleavage, and/or topossible differences in aminolano3v-z- necrea ceut ex-dnucleocapsidswere
re-serum.After the
precipi--ated by SDS-polyacryl-,elsectionscontainingthe )lacedacrossthetopofa
-lab. The gel sectionswere
agaroseandwere
over-ase(1ug).Electrophoresis conductedatlow voltage
toallowpartialdigestioninthestackinggel,anda
fluorograph wasprepared. The gel segments used containedintracellularp40andp80 fromcellextracts
(A) and thep40 and p45proteins from disrupted nucleocapsids (B). Autoradiographs of gelsegments containing theprecipitatedproteinsservedas
refer-encesforthesecond-dimension electrophoresis step (top).80K, 80,000 molecularweight.
J. VIROL.
on November 10, 2019 by guest
http://jvi.asm.org/
A B C
p40 p80 p40 p80 p40 p80
40K
-28K- * -28K
18 K-
-17K-
_4
-17K 4fl14K-_j_
1 1K- -1 1K ,,§W.
8iK-
-FIG. 7. Peptide maps of HSV-2 intracellular p40 and p80. Peptide mapping was performed as described in the text, except thatthe gel segments from the first electrophoresis step were oriented so that the bands were perpendiculartothe directionof the second electrophoresis step. The purified proteins were partially digested with0.1,ug(A),0.5pg(B), and1pg(C) of S. aureus V8 protease. 40 K, 40,000 molecular weight.
P156
acid sequences in portions of the polypeptide
A
chains. The
peptide
mapping
studies indicate
p12
that the
predominant polypeptide species of
in-tracellular
p40 is
closely related
tonucleocapsid
p40 and p45.
However, intracellular p40 also
possesses
polypeptides
which
arestructurally
p40
different from
nucleocapsid
p40, since under the
sameconditions of
partial proteolytic
digestion,
the
digest of intracellular p40 contained peptides
that
wereabsent in the
nucleocapsid
p40
and
p45
digests. The
reasonsfor the appearance of
p15
these peptides have not yet been determined.
B
Little is known about the biochemistry of
E
herpesvirus
assembly.
Studiesemploying
elec-E
tronmicroscopy
indicate that
nucleocapsids
areassembled in the nucleus and
arethen
enveloped
en
I A
I
Aduring
passagethrough
the nuclear membrane
toform complete infectious particles, which are
c
found in the
cytoplasm
(5).
Although
the
func-tion of
intracellular p80 remains
unknown,
webelieve that intracellular p40 participates in
vi-p40 rus
assembly,
since the
p40
and
p45 proteins
areC
I
major constituents of intranuclear
nucleocap-arations immunoprecipitated by guinea pig antise-rum againstHSV-1 nucleocapsidp40. Virions and nucleocapsids werepurified from cells labeled with
p4s5
[35S]methionine
between 18 and 22 h after HSV-1 infection. The labeled virus particles (100,000 cpm) weredissociated andreacted withguinea pig anti-serumagainstHSV-I nucleocapsidp40. Proteinsofnucleocapsids,virions, and immunoprecipitateswere
D separated by electrophoresis. Scanning densitometer
p40 tracings of autoradiograms and
fluorograms
were prepared. Autoradiogram tracings show polypeptides of HSV-1 nucleocapsids (A) and virions (B) aftera 45 13-day exposure. Fluorogram tracings show polypep-tides precipitated by guinea pig antiserum against HSV-1 nucleocapsidp40from disrupted HSV-I nu-FIG. 8. SDS-polyacrylamide gel electrophoresis of cleocapsids aftera 13-day exposure(C) and virions polypeptides of HSV-I nucleocapsidandvirion prep- aftera40-dayexposure(D).
. . . .
on November 10, 2019 by guest
http://jvi.asm.org/
[image:8.510.110.401.68.206.2] [image:8.510.57.253.262.632.2]652 ZWEIG ET AL.
sids. The
questions
of thepresence
ofnucleocap-sid p40
andp45
in virionparticles
and their roles in virus assembly are currently being studied inthis
laboratory.
ACKNOWLEDGMENTS
WethankM.Chakrabartyand L. Newman for excellent technical assistance and J. E. Elser and M. A. Gonda for expert assistance with electronmicroscopy.
This work was supported under Public Health Service contractN01-CO-75380 with the National Cancer Institute.
LITERATURE CITED
1. Bonner, W.M., and R. A. Lasky. 1974. A film detection method fortritium-labeledproteins and nucleic acids in polyacrylamidegels. Eur. J. Biochem. 46:83-86. 2.Bordier, C., and A. Crettol-Jarvinen. 1979. Peptide
mapping of heterogeneous protein samples. J. Biol. Chem. 254:2565-2567.
3. Cassai, E.N., M.Sarmiento,and P. G.Spear. 1975. Comparisonof the virionproteinsspecifiedby herpes simplexvirus types1and 2. J. Virol. 16:1327-1331. 4. Cleveland, D. W.,S. G. Fischer,M. W.Kirschner,
and U. K. Laemmli.1977.Peptide mapping by limited proteolysisin sodiumdodecylsulfate andanalysis by gelelectrophoresis.J.Biol. Chem. 252:1102-1106. 5.Darlington,R.W.,and H.L.Moss.1969.Theenvelope
ofherpesviruses. Prog. Med. Virol.11:16-45. 6.Gibson, W., and B.Roizman. 1972.Proteinsspecified
by herpes simplex virus. VIII. Characterization and composition ofmultiplecapsidforms ofsubtypes1and 2.J.Virol. 10:1044-1052.
7. Heilman, C. J., Jr., M. Zweig, J. R. Stephenson, and B.Hampar.1979.Isolation of a nucleocapsid polypep-tide of herpes simplex virus types 1 and 2 possessing immunologically type-specific and cross-reactive deter-minants. J. Virol.29:34-42.
8. Hutchinson, M. A., T. Hunter, and W. Eckhart. 1978. Characterization of T antigens in polyoma-infected and transformedcells. Cell 15:65-77.
9. Lostrum, M. E., M. R. Stone, M. Tam, W. N. Burnette, A. Pinter, and R. C. Nowin8ki. 1979. Monoclonal antibodiesagainst murine leukemia viruses: identifica-tion of six determinants onthe p15(E) and gp7O enve-lope proteins. Virology 98:336-350.
10.Pauca, E., A.Mellor, R. Harvey, A. E. Smith, R. M. Hewick, and M. D. Waterfield. 1978.Largeandsmall tumor antigens from simian virus 40 have identical aminoterminimapping at 0.65 map units. Proc. Natl. Acad.Sci. U.S.A. 75:2165-2169.
11. Simmons, D. T., and M. A. Martin. 1978. Common methionine-tryptic peptides near the amino-terminal end ofprimate papovavirus tumor antigens. Proc. Natl. Acad. Sci. U.S.A.75:1131-1135.
12. Spear, P.G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpes virion. J. Virol. 9:143-159. 13. Zweig, M.,C. J. Heilman, Jr., and B. Hampar. 1979.
Identification of disulfide-linked protein complexes in the nucleocapsids of herpes simplex virus type 2. Virol-ogy 94:442-450.
14. Zweig, M., C. J. Heilman, Jr., H. Rabin, R. F. Hopkins M,R. H.Neubauer,and B. Hampar. 1979. Produc-tionof monoclonal antibodies against nucleocapsid pro-teins of herpessimplex virus types 1 and 2. J. Virol. 32: 676-678.
J. VIROL.
![FIG.1.pigprepared.precipitatedguinea[35S]methionine2HSV-2immunoprecipitates,E)lanesThe nucleocapsid and SDS-polyacrylamide gel electrophoresis of soluble cell extract proteins immunoprecipitated by pig antiserum against HSV-2 nucleocapsid p40](https://thumb-us.123doks.com/thumbv2/123dok_us/1491186.101791/3.510.88.403.72.295/pigprepared-precipitatedguinea-immunoprecipitates-nucleocapsid-polyacrylamide-electrophoresis-immunoprecipitated-nucleocapsid.webp)