Summary
Stem cell therapy for retinal disease is under way, and several clinical trials are currently recruiting. These trials use human embryonic, foetal and umbilical cord tissue-derived stem cells and bone marrow-derived stem cells to treat visual disorders such as age-related macular degeneration, Stargardt’s disease and retinitis pigmentosa. Over a decade of analysing the developmental cues involved in retinal generation and stem cell biology, coupled with extensive surgical research, have yielded differing cellular approaches to tackle these retinopathies. Here, we review these various stem cell-based approaches for treating retinal diseases and discuss future directions and challenges for the field.
Key words: A ge-related macular degeneration, Retinitis pigmentosa, Stargardt’s disease, Stem cells, Clinical trials
Introduction
Although the retina is a complex structure, many diseases of the outer retina can be attributed to the degeneration of a relatively simple epithelial monolayer: the retinal pigment epithelium (RPE). Although the RPE is not a defined part of the retina, it is an essential supporting tissue involved in retinol cycling, nutrient transport, growth factor production and phagocytosis of the fragile photoreceptor (PR) outer segments (OSs). Much work in the past decade has thus focused on the replacement of this monolayer, initially with an autologous cell source and more recently using stem cells. Using both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), several research groups have been able to guide differentiation into a cell that displays many characteristics and morphological similarities to the RPE cell. This approach, coupled with extensive surgical experience from routine retinal surgery and the development of skills from experimental retinal surgery, has made stem cell-derived RPE cells ideal candidates for transplantation into retinae for the treatment of degenerative diseases. These include diseases such as age-related macular degeneration (AMD), Stargardt’s disease (STGD) and retinitis pigmentosa (RP), for which there are currently no curative treatments.
There are currently several phase I clinical trials in progress using stem cell-based therapies to treat retinal disease, and thus the eye is one of the first organs to be targeted by regenerative medicine. The results of these trials are keenly anticipated, not only to see whether stem cell therapy is a viable approach but also to assess the immune tolerance of stem cell therapy. An alternative
approach to using differentiated stem cells to replace the degenerated tissue directly is to transplant only partially differentiated stem cells or even the stem cells themselves, which can produce trophic factors that rescue the dying cells and restore function. In line with this, umbilical tissue-derived stem cells, foetal stem cells and autologous bone marrow-derived stem cells are being transplanted to the eye in an attempt to maintain a healthy retina in AMD.
In this Review, we discuss the major current issues in retinal stem cell transplantation. We first provide a brief commentary of retinal diseases, previous surgical strategies and relevant preclinical advances in order to contextualise current events. We next discuss the various sources of stem cells that are being applied to treat retinal diseases. The final part of this Review outlines the future applications and obstacles to the ideal retinal stem cell therapy.
A brief overview of stem cells
Stem cells have the ability to differentiate into several cell types but they are also capable of indefinite self-renewal in their undifferentiated state. Stem cells may be classified according to their potency: that is, the range of cell types they can differentiate into. Omnipotent stem cells can differentiate into embryonic and extra-embryonic tissue, pluripotent cells have the ability to only form embryonic tissue (ectoderm, mesoderm and endoderm), and multipotent stem cells are able to differentiate into a limited number of cell types, dictated by a degree of previous differentiation.
Stem cells may also be classified according to their origin. In humans, ESCs are harvested from the inner cell mass of the blastocyst of 5-day-old preimplantation embryos and are pluripotent (Thomson et al., 1998). Foetal stem cells are harvested from foetal tissue and are considered multipotent. Adult stem cells have also been characterised in most major organs, including the eye (Singhal et al., 2012). A rich source of adult stem cells is the bone tissue, which contains both haematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs), housed in the marrow and the stroma, respectively. Umbilical cord tissue is another source of multipotent stem cells that can develop into a variety of cell types. The therapeutic application of stem cells is based on a variety of strategies, the most well-known of which is cell replacement therapy. Here, the stem cells are differentiated into the desired cell type, which is then delivered to the damaged tissue in order to integrate and restore function. An alternative method is via a paracrine effect, whereby the transplanted stem cells secrete trophic factors that induce the resident tissue to self-restore and proliferate (Baglio et al., 2012). Additionally, there is some evidence that stem cells may fuse with individual existing cells in order to restore function (Ying et al., 2002).
Retinal disease candidates for stem cell-based regeneration
The retina is an ideal target for stem cell therapies for a number of reasons. The eye is easily accessible and there is a wealth of Development 140, 2576-2585 (2013) doi:10.1242/dev.092270
© 2013. Published by The Company of Biologists Ltd
Stem cells in retinal regeneration: past, present and future
Conor M. Ramsden1,2, Michael B. Powner1, Amanda-Jayne F. Carr1, Matthew J. K. Smart1, Lyndon da Cruz1,2 and Peter J. Coffey1,3,*
1The London Project to Cure Blindness, Division of ORBIT, Institute of
Ophthalmology, University College London, 11-43 Bath Street, London, EC1V 9EL, UK. 2NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London, EC1V 2PD, UK. 3Center for Stem Cell Biology and Engineering, NRI, UC Santa Barbara, CA 93106, USA.
*Author for correspondence (p.coffey@ucl.ac.uk)
D
E
V
E
LO
P
M
E
N
surgical expertise in dealing with the retina, which is facilitated by the transparent cornea. This transparency also aids clinical assessment following intervention and allows documentation with photography and other imaging modalities. The blood-retina barrier confers a degree of immune privilege in the healthy eye, but whether this is of any benefit to transplants in diseased eyes remains to be seen. Furthermore, although we are dependent on sight for a normal life, the eye is not essential to sustain life. This allows full characterisation for the life of the graft, even if the recipient organ continues to fail. Finally, many of the diseases of the retina are very well characterised.
The retina is a complex multilayered tissue that relays information from light energy and converts it to electrical energy that is transmitted to the brain to build up an image of the visual environment. Juxtaposed against the outermost layer of the retina is the retinal pigment epithelium (RPE, Fig. 1), a support tissue for the highly metabolic PRs of the outer retina. Light is converted to electricity within the PRs. From here, the information is transmitted inwards via the bipolar cells and finally to the ganglion cells, which synapse in the superior colliculus and deliver the information in a retinotopic fashion.
The normal RPE is a polarised monolayer that is derived from the neuroepithelium of the anterior neural plate in the developing embryo. It is a highly metabolically active cell layer with numerous and varied functions that act to support the fragile PR cell layer. The primary functions of the RPE include the transport of nutrients, recycling of retinol, production of pigment and the phagocytosis of cone and rod OSs. The polarised RPE secretes pigment
epithelium-derived growth factor (PEDF) from its apical side and vascular endothelial growth factor (VEGF) from its basal side. Tight junctions between neighbouring cells form the blood-retinal barrier and each cell adheres to Bruch’s membrane (Strauss, 2005).
The PR and associated retinal circuitry is indeed a complex system that would be challenging to re-engineer through regenerative medicine. A much more straightforward target that has emerged in recent years is the RPE (Strauss, 2005). There are several diseases that affect the RPE, leading to RPE damage (Fig. 1B) and hence loss of vision. A detailed discussion of all of these RPE degenerations lies outside the scope of this Review and the focus here will be on AMD, STGD and RP, as these are the first targets of stem cell therapy in the retina.
Age-related macular degeneration
Age-related macular degeneration is the leading cause of irreversible blindness in the over 55 age group worldwide (de Jong, 2006). Damage to the macular area of the central retina results in loss of the central visual field (Fig. 2A,B). Advanced AMD may be labelled as dry (geographic atrophy, GA) or wet (choroidal neovascularization, CNV) based on the absence or presence, respectively, of fluid at the macula. Almost two-thirds of over 80-year olds have at least early signs of AMD. In the UK alone, there are half a million people with late AMD and 1.75 million in the USA, highlighting the massive burden of disease that is set to almost double in the next 10 years (Owen et al., 2012; Friedman et al., 2004). Furthermore, once late AMD has been diagnosed in one eye, up to 42% of patients develop late AMD in the fellow eye, and 12% will be registered blind within 5 years (Jager et al., 2008).
AMD is a complex degenerative disease with a polygenic hereditary component (Haines et al., 2005; Churchill et al., 2006). It arises as the result of chronic, low grade inflammation in the central outer retina, which leads to degeneration of the RPE and its basement membrane, Bruch’s membrane (Jager et al., 2008) (Fig. 1B). Under normal conditions, each RPE cell is responsible for the phagocytosis of millions of PR OS discs (Sparrow et al., 2010). Over time, the incomplete digestion of each phagosome results in accumulation of the lysosomal protein lipofuscin, which is toxic to RPE cells. In addition, Bruch’s membrane doubles in thickness between 10 and 90 years of age, slowing the import of nutrients and the export of waste from the RPE, and impairing the ability of the RPE to adhere to its basement membrane (de Jong, 2006). In the case of dry AMD, this gradual deterioration of RPE health leads to subsequent PR loss at the macula. In wet AMD, fluid accumulation occurs as the result of unwanted neovascular membranes growing from the choroid through Bruch’s membrane and the RPE into the subretinal space and occasionally through the retina. This neovascularisation is driven by the presence of excess VEGF on the apical side of the RPE, which promotes the growth of fenestrated, leaky capillaries that allow the build up of fluid. Occasionally, these fragile vessels cause haemorrhage at the macula, which may result in scarring (Jager et al., 2008).
Stargardt’s disease
Stargardt’s disease is an inherited disease of the retina. It is the most common cause of macular disease in children, with an incidence of 1:10,000 live births, and it presents in the first and second decades of life. The disease progresses to leave subjects legally blind as adults as the central vision fades (Fig. 2A,B). The clinical picture is of yellow flecks in the macular region that correspond to the abnormal build up of the waste pigment lipofuscin in the PRs, and subsequently the RPE, after PR A Healthy RPE
B Degenerated RPE
OS
OS Ph
RPE
RPE BM
[image:2.612.53.289.388.649.2]BM
Fig. 1. Healthy and degenerated retinal pigment epithelium.
(A) Healthy retinal pigment epithelium (RPE) also showing the outer segments (OS, grey) of the photoreceptors. (B) Degenerated RPE is discontinuous, shows the loss of adherence (yellow bars) to Bruch’s membrane (BM, green) and the loss of tight junctions (red bars) between RPE cells. Photoreceptor loss also occurs due, in part, to the inability of the degenerated RPE to phagocytose the photoreceptor outer segments,
as illustrated by the lack of phagosomes (Ph) within the RPE cells.
D
E
V
E
LO
P
M
E
N
phagocytosis (Walia and Fishman, 2009). The most common form of the disease is STGD1, which is autosomal recessive. It is caused by mutations in the ABCA4gene (Allikmets et al., 1997), which encodes a transporter protein that is normally expressed on the PR OSs. The less common forms of STGD are: STGD3, caused by mutations in the ELOVL4gene (Zhang et al., 2001), which codes for a protein involved in the production of fatty acids in the PRs; and STGD4, caused by defects in the PROM1gene, which codes for a PR transmembrane glycoprotein (Yang et al., 2008). Although there is a wealth of information and valuable research into the molecular genetics of STGD, there are currently no recognised treatments for the disease.
Retinitis pigmentosa
Retinitis pigmentosa refers to a group of inherited retinal degenerations that mostly affect the rod visual system. There are over 100 defined genetic mutations that may lead to RP, and it may be inherited in a dominant, recessive or X-linked fashion. As such, it has a very variable clinical course, though most patients report problems with night blindness and progressive peripheral visual field loss, leading to tunnel vision (Fig. 2C), which is often followed by blindness (Hartong et al., 2006). In many cases, RP progresses to involve the central visual field. The classical clinical picture is of pigment deposition in the peripheral retina. Of the 1 in 4000 people affected, the most common RP subtype occurs due to mutations in the gene encoding rhodopsin and accounts for 30% of autosomal dominant cases. Although many of RP mutations code for genes in PRs, many RP subtypes begin with primary failure of the RPE. The RPE-specific genes RPE65(Hamel et al., 1994) and MERTK(Gal et al., 2000), among many others, are also implicated in RP and could be ideal targets for replacement with stem cell-derived RPE.
Retinal transplantation in AMD: proof of principle Currently, there is no treatment that can reverse dry AMD, although dietary supplementation with defined vitamins and antioxidants has been shown to slow progression (Age-Related Eye Disease Study Research Group, 2001). In the case of wet AMD, intravitreal injection of anti-VEGF agents can stabilise the disease, halting the decline of visual acuity in 90% of individuals with a subtype of wet AMD (Martin et al., 2011; Rosenfeld et al., 2006). However, this treatment is expensive and must be administered frequently in order to have any positive effect. Furthermore, the long-term side-effects and potential benefits of this relatively new therapy are yet to be elucidated.
With no curative treatments available for either type of AMD, several surgical approaches to reverse the pathology have been attempted over the past 20 years. Surgical techniques were first developed while performing submacular surgery to remove neovascular membranes and haemorrhage. A systematic review (Giansanti et al., 2009) suggested that submacular surgery was of
no benefit in wet AMD and, as with all procedures requiring a vitrectomy, there was an increased rate of cataract, retinal detachment and proliferative vitreoretinopathy.
Given that the diseased RPE is a major component of AMD, attempts have been made to replace the RPE at the macula, either by moving the macula to the non-diseased periphery or by grafting new RPE under the macula. Sources of RPE cells that have been used in these procedures include rotated pedicled flaps of peripheral RPE-choroid, autologous free RPE-choroid grafts, sheets of foetal RPE and cell suspensions of peripheral RPE (Binder et al., 2007; da Cruz et al., 2007; Chen et al., 2009; van Zeeburg et al., 2012). In the last approach, one of the major drawbacks is that there is no guarantee that the cells in suspension can first attach to the diseased Bruch’s membrane (Tsukahara et al., 2002). Moreover, those cells that attach often do not form the desired monolayer that is required for optimal RPE function, and they instead clump into rosettes (Vugler et al., 2008) or undergo anoikis (Tezel et al., 2004), a form of apoptosis specific to anchorage-dependent cells that are dissociated from their usual extracellular matrix.
Macular translocation and RPE transplantation are complex, lengthy and expensive procedures that often require further surgery to address complications such as unplanned retinal detachment, cataracts and double vision. It is unlikely that these procedures will prove cost effective in combatting the huge burden of AMD. A further constraint of autologous RPE transplant is that it is difficult to collect enough material to repopulate the entire macula adequately. Moreover, the cells being harvested are the same age as the cells they are intended to replace. It is estimated that the macula harbours 60,000 RPE cells that potentially need replacing. To overcome this, it will be necessary to develop new techniques to derive and expand RPE cells in vitro, or to use the paracrine effects of stem cells to trigger rejuvenation.
Potential sources of cells to treat retinal disease The ideal cell-based therapy for RPE regeneration would have high efficacy, a low failure rate, be reproducible across a range of phenotypes and not require immunosuppression. It is preferable that the tissue is easy to propagate, requiring a low harvest rate and with few ethical issues. It should also cost little and require few resources, and both the culture and transplantation of the tissue should not be technically difficult to execute. Potential sources of such cells for cellular therapy of AMD are wide ranging and include both pluripotent stem cells and multipotent stem cells of foetal and adult tissues.
Human embryonic stem cells
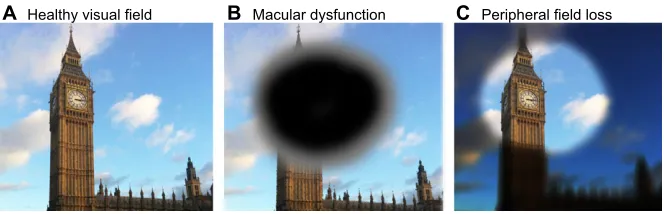
The first account of the rescue of visual function by primate ESCs (by replacement of the RPE monolayer) was that of cynomolgus monkey ESC-derived RPE transplanted into Royal College of Surgeon rats (the RCS rat, see Box 1), an accepted animal model for AMD (Haruta et al., 2004). Since this time, two main A Healthy visual field B Macular dysfunction C Peripheral field loss Fig. 2. The effects of retinal pigment
epithelium damage on visual field. (A) The visual fields in normal subjects. (B) The visual field in the case of macular diseases, such as age-related macular degeneration and Stargardt’s disease. (C) The tunnel visual field experienced in individuals with retinitis pigmentosa.
D
E
V
E
LO
P
M
E
N
[image:3.612.51.383.60.167.2]approaches to propagate RPE-like cells from human ESCs (hESCs) have been described, either spontaneous or directed. The former method allows the overgrowth of stem cell colonies, which results in spontaneous differentiation of RPE cells following removal of fibroblast growth factor (FGF) from the culture medium (Klimanskaya et al., 2004; Lund et al., 2006; Vugler et al., 2008). After a few weeks, pigmented areas of cells develop, from which the like cells are derived. Spontaneous differentiation to RPE-like cells can also be achieved using embryoid bodies grown in suspension that are plated out as adherent colonies in neural differentiation medium (Meyer et al., 2009).
A more-directed approach can be achieved by blocking the Wnt and Nodal signalling pathways with dickkopf 1 (DKK1) and left-right determination factor (LEFTY) (Ikeda et al., 2005), respectively. Alternative methods include incubation sequentially with nicotinamide and then activin A (Idelson et al., 2009). Although yield and speed are greater with the directed methods of RPE differentiation, there may be benefits of using a more spontaneous method. Exogenous factors used to drive stem cells down a neural and eye field pathway are expensive, and the associated cell culture techniques are laborious. Furthermore, factors such as DKK1 and LEFTY are often derived from bacterial and animal sources, which can add a layer of complexity to the regulatory process for human therapies.
Human ESC-derived RPE appears to replicate very closely the endogenous tissue morphology and function. Analysis of the hESC-derived RPE grown as a monolayer revealed a carpet of hexagonal cells with apical microvilli and pigment-containing melanosome granules (Fig. 3). Furthermore, the hESC-derived RPE cells displayed polarity with the correct apical orientation of Na+K+ATPase and expressed proteins associated with tight junctions (Vugler et al., 2008). Gene and protein expression analysis of the hESC-derived RPE in vitroshowed expression of proteins involved in retinol cycling, as well as expression of RPE65, a protein specific to RPE cells. In addition, hESC-derived RPE cells express PAX6 at levels similar to foetal RPE cells
(Klimanskaya et al., 2004); furthermore, PEDF is preferentially secreted from their apical surface and the tight junction protein 1 (ZO1) is expressed only on their cell borders (Zhu et al., 2011). Finally, phagocytosis was observed in assays in vitroand following transplantation into RCS rat eyes (Carr et al., 2009; Idelson et al., 2009; Vugler et al., 2008), confirming that these cells are indeed RPE like.
With the aim of translating these studies into clinical trials, hESC-derived RPE has been transplanted into the subretinal space of RCS rats and has been shown to slow the degeneration of PRs, improve visual performance and sustain objective measures of retinal electrical activity (Lu et al., 2009; Lund et al., 2006; Vugler et al., 2008). To assess safety, hESC-derived RPE has also been transplanted into NIH III mice, which are immune deficient, and devoid of natural killer cells and mature T and B cells (Lu et al., 2009). These mice will normally display a reduced ability to fight off infection and combat cancer, and are thus ideal candidates for assessing tumour formation. Of the 45 animals that received a transplant, none developed teratomas or displayed any other evidence of dysplasia throughout their life. This is encouraging, although caution must be exercised as different groups have reported tumour formation in up to 50% of mice that received hESC-derived neural precursors transplanted to the subretinal space (Arnhold et al., 2004). In this case, however, the neural precursor cells are only partially differentiated and thus may be more tumourigenic than the terminally differentiated hESC-derived RPE cells. Although RPE can be cultured and expanded in vitro (Salero et al., 2012), in a healthy human macula it is generally regarded to be post mitotic. As pluripotent stem cell-derived RPE cells display many structural and functional features of native RPE, it is expected that they will not proliferate, especially if transplanted in their natural monolayer morphology.
In summary, hESC-derived RPE cells display many of the characteristics of native RPE, including morphology, polarity, phagocytosis, tight junctions and retinol cycling. After transplantation into animal models of AMD, hESC-derived RPE cells can rescue visual parameters and could represent the ideal source for cell therapy for AMD.
The preliminary results of a clinical trial in which a suspension of dissociated hESC-derived RPE cells was transplanted to two Box 1. The RCS rat
The Royal College of Surgeons (RCS) rat is a commonly used animal model for retinal pigment epithelium (RPE) diseases, including age-related macular degeneration (AMD), and is phenotypically characterised by the build up of phagosomes on Bruch’s membrane, akin to AMD. These rats harbour a mutation in their Mertk gene (D’Cruz et al., 2000), which encodes MERTK, a transmembrane tyrosine kinase that is expressed in the RPE and plays a role in the signal transduction pathway involved in the phagocytosis of rod outer segments (OSs). In the healthy retina, the stacked phospholipid discs of the rod OSs are renewed at the base by 10% each day. As the discs travels towards the RPE, they generate toxic reactive oxygen species owing to their environment of rapid metabolism and high UV light. These increasingly unstable OS discs are phagocytosed in a circadian rhythm by the RPE. This event is facilitated by adhesion through the apically expressed αVβ5 integrin protein and subsequent transduction to initiate the phagocytosis process via MERTK.
Extrapolating data from RCS rats into humans is difficult. Rats have a much lower density of cones and entirely lack maculae. Additionally, the RCS rat has a young Bruch’s membrane, unlike AMD pathology. Indeed, experiments in which retinal detachments in RCS rats have been induced by saline alone have rescued some visual response in these rats, presumably by ‘cleaning’ the debris from the RPE/Bruch’s complex (Sauvé et al., 2002).
ZO1/connexin 43
Nuc
[image:4.612.320.557.511.640.2]B
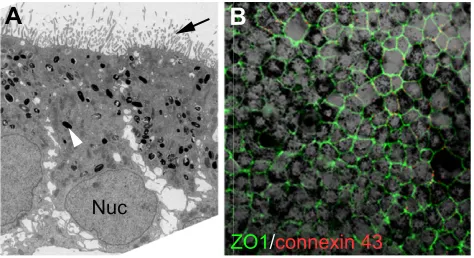
A
Fig. 3. Phenotypic characterisation of human embryonic stem cell-derived retinal pigment epithelium.(A) Electron microscopy of human embryonic stem cell (hESC)-derived retinal pigment epithelium (RPE) showing features typical of native polarised RPE, such as basal nucleus (Nuc) location, presence of melanocytes (white arrowhead) and apical microvilli (black arrow). (B) Immunocytochemistry showing the pigmented monolayer of hESC-derived RPE cells with hexagonal shape, as demarcated by the tight junction protein ZO1 in green and the
intercellular gap junction protein connexion 43 in red.
D
E
V
E
LO
P
M
E
N
individuals (one with dry AMD and one with STGD) were reported in 2012 by Advanced Cell Technology (ACT), California, USA (Schwartz et al., 2012). The cells, developed by ACT and termed MA09-hRPE, were injected into the submacular space following a vitrectomy procedure. Four months after transplantation, there was no evidence of teratoma formation and no loss of vision in either individual. It was suggested that new pigmentation near the injection site in the patient with STGD was evidence of the RPE function. More results are awaited from this multicentre trial (see Table 1 for a summary of this and other trials using stem cells for retinal disease).
ESC-derived RPE cells have been shown to have the morphology and essential function of native RPE, and may be an ideal source of cells for transplantation. Their source is, however, ethically contentious; thus, other types of stem cells are being considered for retinal repair in AMD and other retinal degenerations. Although replacement of the degenerated RPE is a worthwhile strategy when this layer has been lost, in earlier forms of the disease the simple rescue of the failing, but still present, RPE may be enough to reverse pathology.
Human foetal stem cells
Foetal stem cells are derived from a wide variety of embryonic and extra-embryonic tissues and are regarded as distinct from adult stem cells and ESCs (Pappa and Anagnou, 2009). Recently, human foetal neural stem cells were isolated from enzymatically dissociated brain tissue of donated aborted foetuses (16-20 weeks
gestation). The stem cells were identified by their expression of the cell-surface maker CD133 and were expanded in cell culture conditions that had been optimised for neural cell culture to induce rosette formation. Once dissociated, these suspensions of neuralised foetal stem cells were injected into the subretinal space of the RCS rat (McGill et al., 2012), where they survived and migrated throughout the retina and away from the injection site. Basic visual parameters and retinal histology were improved in the treated rats, though the exact mechanism of repair is unknown as the transplanted cells do not adopt a retinal morphology or express retinal markers. These cells, labelled HuCNS-SC, have been developed by Stem Cells Inc. (Newark, CA, USA). More recently, Stem Cells, in partnership with the Retina Foundation of the Southwest (Dallas, TX, USA), have initiated a clinical trial, initially in 16 patients with dry AMD, using a sub-retinal injection of stem cells. The results of this trial are awaited.
Human umbilical tissue-derived stem cells
[image:5.612.52.561.389.699.2]Human umbilical tissue harbours multipotent stem cells that are classified as adult stem cells. Such stem cells can be retrieved from donor umbilical cords through enzymatic digestion and can be expanded in cell culture. Suspensions of these human umbilical tissue-derived stem cells (hUTSCs) were injected subretinally into 80 RCS rats (Lund et al., 2007), which resulted in better outcomes in terms of the visual parameters compared with controls. The mechanism of repair observed is thought to be paracrine, as the hUTSCs do not change their morphology and were found to secrete
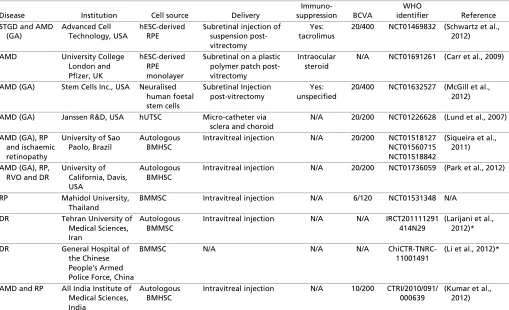
Table 1. Clinical trials using stem cells for retinal disease that are currently registered on WHO clinical trial register
Disease Institution Cell source Delivery
Immuno-suppression BCVA
WHO
identifier Reference STGD and AMD
(GA)
Advanced Cell Technology, USA
hESC-derived RPE
Subretinal injection of suspension post-vitrectomy
Yes: tacrolimus
20/400 NCT01469832 (Schwartz et al., 2012)
AMD University College London and Pfizer, UK
hESC-derived RPE monolayer
Subretinal on a plastic polymer patch post-vitrectomy
Intraocular steroid
N/A NCT01691261 (Carr et al., 2009)
AMD (GA) Stem Cells Inc., USA Neuralised human foetal stem cells
Subretinal Injection post-vitrectomy
Yes: unspecified
20/400 NCT01632527 (McGill et al., 2012)
AMD (GA) Janssen R&D, USA hUTSC Micro-catheter via sclera and choroid
N/A 20/200 NCT01226628 (Lund et al., 2007)
AMD (GA), RP and ischaemic retinopathy
University of Sao Paolo, Brazil
Autologous BMHSC
Intravitreal injection N/A 20/200 NCT01518127 NCT01560715 NCT01518842
(Siqueira et al., 2011)
AMD (GA), RP, RVO and DR
University of California, Davis, USA
Autologous BMHSC
Intravitreal injection N/A 20/200 NCT01736059 (Park et al., 2012)
RP Mahidol University, Thailand
BMMSC Intravitreal injection N/A 6/120 NCT01531348 N/A
DR Tehran University of Medical Sciences, Iran
Autologous BMMSC
Intravitreal injection N/A N/A IRCT201111291 414N29
(Larijani et al., 2012)*
DR General Hospital of the Chinese People’s Armed Police Force, China
BMMSC N/A N/A N/A ChiCTR-TNRC-11001491
(Li et al., 2012)*
AMD and RP All India Institute of Medical Sciences, India
Autologous BMHSC
Intravitreal injection N/A 10/200 CTRI/2010/091/ 000639
(Kumar et al., 2012)
AMD, age-related macular degeneration; BCVA, best corrected visual acuity; BMHSC, bone marrow-derived haematopoeitic stem cell; BMMSC, bone marrow-derived mesenchymal stem cell; DR, diabetic retinopathy; GA, geographic atrophy; hESC, human embryonic stem cell; hUTSC, human umbilical tissue-derived stem cells; N/A, information not available; RP, retinitis pigmentosa; RPE, retinal pigment epithelium; RVO, retinal vein occlusion; STGD, Stargardt’s disease.
*References are not specific to retinal disease.
D
E
V
E
LO
P
M
E
N
high levels of brain-derived neurotrophic factor, which is known to improve neuronal survival. Building on these findings, Janssen Research and Development (Philadelphia, PA, USA), a research arm of Johnson and Johnson, have started a clinical trial of a hUTSC cell line, termed CNTO 2476, in individuals with dry AMD. These cells are delivered using a catheter delivery system through the white fibrous sclera and the vascular choroidal tissue to the subretinal space. There are no results from this phase I/II trial as yet.
Bone marrow-derived HSCs
In mice, it has been shown that endogenous HSCs migrate to the subretinal space in damaged retinae (Li et al., 2006), presumably to initiate repair. Allogenic bone marrow-derived HSCs (BMHSCs) have been delivered via the tail vein to mice shortly after experimentally induced damage to the retina (Atmaca-Sonmez et al., 2006). These exogenous cells were shown to migrate to the retina, proliferate and express RPE65 (an RPE-specific protein). This expression of RPE protein suggests that these transplanted cells are reacting appropriately to their new niche; however, the expression of RPE65 alone is not indicative of their attaining RPE function. RPE cells have typical morphology and many essential functions, all of which would need to be characterised in the transplanted cells in order to be confident that this is a viable RPE replacement. One group (Otani et al., 2004) showed improved visual parameters in a mouse model of RP following intravitreal injection of such BMHSCs. In humans, intravitreal injection of BMHSCs into three individuals with RP was shown to have no adverse side effects, although visual parameters in these cases did not improve significantly (Siqueira et al., 2011). The same group is currently recruiting to use this therapy on subjects with AMD and vascular retinopathies such as retinal vein occlusion and diabetic retinopathy, both of which result in retinal ischaemia. In California, another group is recruiting for a trial that uses intravitreal injection of autologous BMHSCs to treat retinal vein occlusion, following their use in animal studies in which they were deemed to be safe (Park et al., 2012).
Bone marrow-derived MSCs
Bone marrow-derived MSCs (BMMSCs) also hold promise as a source for cellular therapy in diseases of the RPE. These cells were injected subretinally in mouse models of RP (Arnhold et al., 2007), whereupon RPE and PR morphology appeared to improve. BMMSCs were also injected into the subretinal space of the RCS rat (Lu et al., 2010), where they were found to reverse the decline of basic visual parameters. Of note, in the RCS rat, BMMSCs displayed no difference in graft survival regardless of immunosuppression, suggesting that mesenchymal cells are potentially less immunogenic than other sources of allogenic stem cells.
Future applications and directions
Regenerative stem cell-based therapies may become the standard means of treating the huge burden of severe retinal degeneration, but it is still far off routine clinical practice. Although these first trials will help answer the most pressing question of efficacy, there are many issues that need to be resolved. Embryonic-derived stem cells appear to be able to form cells with the structure and many of the functions of RPE but their origin is an ethically contentious issue, as with foetal brain-derived stem cells. Current methods for transplantation using cell suspensions are not likely to develop into monolayers and there are likely to be issues with immune rejection.
Although autologous bone marrow-derived stem cells are likely to be less immunogenic after transplantation, it remains to be seen whether any of these modalities have the potential to restore vision. The challenge for the future, then, is to develop an ethically acceptable therapy that restores sight, either by transplanting retinal tissue or by inducing retinal repair, without immunological rejection.
Transplanting hESC-derived RPE as a monolayer
In an attempt to recreate the natural anatomy of the RPE, the London Project to Cure Blindness (LPCB; London, UK) has developed a hESC-derived RPE cell line that can be cultured as a monolayer on a thin sheet of polymer (Carr et al., 2013). This approach aims to overcome the disorganised fashion in which RPE cells adhere to Bruch’s membrane when injected as a suspension (Fig. 4). The plastic polymer is also designed to act as a replacement for the aged and thickened Bruch’s membrane, and provides an anchor for the cells as well as aiding in surgical delivery. A clinical trial with this RPE patch graft is currently recruiting in London.
Using iPSCs
ESC-like cells may also be generated from differentiated somatic cells by reprogramming with multiple factors (Takahashi and Yamanaka, 2006; Fusaki et al., 2009; Warren et al., 2010; Kim et al., 2009). Forced expression of four transcription factors, Oct4, Sox2, Myc and Klf4, can reprogramme these cells to ESC-like cells with pluripotent qualities, termed iPSCs. Other groups have achieved similar results using different combinations of transcriptions factors, such as Oct4, Sox2, Nanog and Lin28, which avoids the use of Myc, a known proto-oncogene (Yu et al., 2007). RPE has also been derived from iPSCs (Buchholz et al., 2009; Carr et al., 2009; Hirami et al., 2009). As with hESC-derived RPE, there are differing protocols used to generate RPE-like cells from iPSCs. The iPSC-derived RPE cells form monolayers that exhibit polarity, form tight junctions, express genes vital to the visual cycle and are able to phagocytose. Transplantation of iPSC-derived RPE into RCS rats has been shown to improve visual function after 13 weeks (Buchholz et al., 2009; Carr et al., 2009; Liao et al., 2010; Meyer et al., 2009; Osakada et al., 2009). One group in Japan (Hirami et al., 2009) is planning a clinical trial using iPSC-derived RPE for AMD. This will be among the first clinical trial using iPSCs in humans.
Diseases that are caused primarily by single gene defects, such as STGD, represent a greater challenge for iPSC-based therapies. This is because iPSCs generated from a diseased individual, as well as their differentiated progeny, will still contain the disease-causing genetic abnormality. Strategies are being developed to genetically correct iPSCs prior to differentiation and transplantation. Proof of principle for this approach comes from Meyer and colleagues, who corrected the mutation in the ornithine aminotransferase gene that causes gyrate atrophy in patient-derived iPSCs (Meyer et al., 2011). The genetically corrected cells were then differentiated into RPE cells, which were observed to have normal levels of ornithine aminotransferase as measured by enzyme assay, compared with the uncorrected gyrate atrophy parental iPSC line (Meyer et al., 2011). In the case of AMD, genetic manipulation may not be necessary as AMD is a polygenic disorder of systemic origin and thus no single gene corrections would provide a total cure. Moreover, the major insult in AMD is wear and tear through years of low grade systemic inflammation, and the expectation is that simply rejuvenating the damaged tissue will be enough to ‘wind back the
clock’ and alleviate the symptoms.
D
E
V
E
LO
P
M
E
N
iPSC-based cellular therapies represent an exciting avenue for regenerative medicine and may herald the dawn of personalised medicine. iPSCs also avoid many of the ethical concerns of embryo-derived stem cells, and furthermore may be matched to the individual. However, there are several issues that must be fully examined before iPSCs may be widely used in clinical practice. Although considered as functionally equivalent to ESCs, iPSCs have been shown to harbour subtle differences in gene expression and DNA methylation (Doi et al., 2009), and thus caution must be taken in extrapolating ESC-based therapies to iPSCs. Furthermore, there have been reports of point mutations and copy number variation in iPSCs (Gore et al., 2011), which raises possible safety issues. It has also been observed that chromosomal telomeres were significantly shortened in iPSC lines compared with native RPE (Kokkinaki et al., 2011). Telomere length is a marker for cell nascence, with shorter telomeres being associated with aging DNA. This suggests that iPSC-based therapies may not have the intended shelf life and more than one therapeutic episode may be required. Finally, there is concern that autologous iPSCs may be immunogenic, though this issue has been somewhat resolved by the work of Araki and colleagues, who showed that this may only be true for pluripotent iPSCs, and not for their terminally differentiated progeny (Zhao et al., 2011; Araki et al., 2013; Guha et al., 2013). An alternative approach to the use of autologous iPSCs is the development of banks of undifferentiated cells that could be differentiated into the desired cell type and transplanted to human leukocyte antigen-matched recipients (Zimmermann et al., 2012)
With the unknown long-term effects coupled with the potential forced expression of known proto-oncogene genes, caution must be exercised before iPSC-based treatment can be extrapolated into the clinical environment. Furthermore, the cost of truly allogenic iPSC therapy, using current technology, would be too high to produce these treatments on the large scale needed to meet the massive demands of a disease such as macular degeneration.
Transplantable PRs: using stem cells as a source
Photoreceptor death in AMD and STGD occurs as a consequence of dysfunction of the diseased RPE. As such, rejuvenating or replacing the RPE is a good place to start. However, when the PRs are lost, the resulting visual deficit is permanent. This is a common occurrence in advanced AMD (wet or dry), STGD and RP. For these individuals, a potential step forward in regaining vision is to replace the degenerated PR cells. This is a viable strategy for AMD as it has been shown that ganglion cells can survive in retinae even when there is severe underlying PR loss (Medeiros and Curcio, 2001). However, finding a suitable source of these transplantable cells is the next step and the limiting factor. Foetal retina is an obvious source of retinal precursors that have the potential to reintegrate into degenerating retina. Retinal precursors isolated from foetal tissue have been transplanted into the subretinal space of both mice and human subjects with PR loss and have improved visual behaviour in the mice (Humayun et al., 2000; MacLaren et al., 2006; Pearson et al., 2012; Radtke et al., 2004). However, it has been demonstrated (MacLaren et al., 2006) that the only viable cells that integrated into the mature retina and differentiated into functional rod PRs were those that were already committed to a PR fate.
When translated from mouse models to use in humans, there are two major drawbacks to this approach: first, the limited supply of foetal tissue; and, second, that the equivalent foetal stage for harvesting the required cells would be after 13 weeks gestation, which leads to significant ethical concerns. Both these issues can be significantly alleviated by using pluripotent stem cells. In 2006, Lamba et al. generated PRs from hESCs-derived embryoid bodies grown in neural culture media supplemented with IGF1, noggin (NOG) and DKK1 (Lamba et al., 2006). Similar protocols, some of which leave out NOG for a period (Banin et al., 2006) or add LEFTY (Mellough et al., 2012; Meyer et al., 2011; Osakada et al., 2009) have also been reported and have differing success rates. Furthermore, PRs have also been generated from mouse and human iPSCs (Hirami et al., 2009).
A
OS
Ph
RPE
BM
B
RPE patch
Degenerated host RPE
PP
C
RPE
OS
PP
6 mm
[image:7.612.52.516.58.265.2]3 mm
Fig. 4. Photoreceptor rescue using human embryonic stem cell-derived retinal pigment epithelium patches.(A) The proposed mechanism of photoreceptor rescue using a retinal pigment epithelium (RPE) patch graft of human embryonic stem cell (hESC)-derived RPE cultured on a plastic polymer substrate (red bracket) that is transplanted between the native, degenerated RPE (blue bracket) and photoreceptor outer segments. (B) The pigmented RPE monolayer on the plastic polymer, ready for implantation. (C) Histological section of a patch graft (red bracket) in a normal porcine eye, lying over the healthy native pig RPE (yellow bracket). BM, Bruch’s membrane; OS, outer segments; Ph, phagosome; PP, plastic polymer; RPE, retinal pigment epithelium.
D
E
V
E
LO
P
M
E
N
More recently, it was shown that culture under conditions of relative hypoxia with supplementation of DKK1, LEFTY, activin A and other factors significantly increased the PR yield from mouse ESCs. The relative hypoxia (2% O2) is closer to tissue normoxia in utero, thus mimicking embryogenesis, which may explain the higher yield (Garita-Hernández et al., 2013).
Despite the observation that PR transplantation alone can restore visual response in a simple mouse disease model (MacLaren et al., 2006), in order to support any PR graft in advanced age-related disease, it is likely that the RPE would also need to be replaced in order to mimic the healthy environment. In line with this, it has been shown that the survival of PR progenitor cells was increased when co-cultured with hESC-derived RPE (Zhu et al., 2011), suggesting that dual replacement is likely to be a promising strategy. Furthermore, recent developments have seen the formation of entire optic cups from both mouse ESCs (Eiraku et al., 2011) and hESCs (Nakano et al., 2012) when grown as aggregates of cells in minimal media conditions. This source of stratified neural retina and RPE in a single complex may also be a potential route to develop a dual RPE/PR graft that can be used in individuals at later stages of the disease with severe neural retina and RPE loss. Although it may be challenging to derive a funtional graft from just these aggregates, they represent an exciting opportunity in disease modelling.
Conclusions
Stem cell transplantation for retinal disease is currently transitioning from over a decade of preclinical research to phase I/II clinical trials. The results of these trials are keenly anticipated, not just regarding efficacy but also to elucidate the levels of immunosuppression required, the difference between RPE replacement and paracrine models, and to determine which delivery method is preferable. Further trials are expected to help determine whether an RPE suspension is adequate or whether an RPE monolayer is required to rescue retinal function. The transplantation of iPSC-derived RPE will be one of the first clinical applications of iPSCs and will help to define how immunogenic iPSCs are in comparison with hESCs. In addition, further preclinical research is under way to develop a source of PRs from stem cells that can be used for neural retinal replacement. The therapeutic application of stem cells for retinal disease has thus begun. Whether this approach will be an effective treatment is the first and most important issue to address.
Acknowledgements
The authors thank Shazeen Hasan and Sakina Gooljar for providing images of the hESC-RPE patch.
Funding
This work was supported by funding from The London Project to Cure Blindness; the Medical Research Council (MRC) UK; the California Institute of Regenerative Medicine (CIRM); Fight for Sight UK; The Lincy Foundation; the Macular Society; and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital National Health Service (NHS) Foundation Trust and University College London Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
References
Age-Related Eye Disease Study Research Group(2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins
C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol.119, 1417-1436. Allikmets, R., Singh, N., Sun, H., Shroyer, N. F., Hutchinson, A.,
Chidambaram, A., Gerrard, B., Baird, L., Stauffer, D., Peiffer, A. et al. (1997). A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet.15, 236-246. Araki, R., Uda, M., Hoki, Y., Sunayama, M., Nakamura, M., Ando, S., Sugiura,
M., Ideno, H., Shimada, A., Nifuji, A. et al.(2013). Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature494, 100-104.
Arnhold, S., Klein, H., Semkova, I., Addicks, K. and Schraermeyer, U.(2004). Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest. Ophthalmol. Vis. Sci.45, 4251-4255.
Arnhold, S., Absenger, Y., Klein, H., Addicks, K. and Schraermeyer, U.(2007). Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefe’s Arch. Clin. Exp. Ophthalmol.245, 414-422.
Atmaca-Sonmez, P., Li, Y., Yamauchi, Y., Schanie, C. L., Ildstad, S. T., Kaplan, H. J. and Enzmann, V.(2006). Systemically transferred hematopoietic stem cells home to the subretinal space and express RPE-65 in a mouse model of retinal pigment epithelium damage. Exp. Eye Res.83, 1295-1302.
Baglio, S. R., Pegtel, D. M. and Baldini, N.(2012). Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol.3, 359.
Banin, E., Obolensky, A., Idelson, M., Hemo, I., Reinhardtz, E., Pikarsky, E., Ben-Hur, T. and Reubinoff, B.(2006). Retinal incorporation and
differentiation of neural precursors derived from human embryonic stem cells.
Stem Cells24, 246-257.
Binder, S., Stanzel, B. V., Krebs, I. and Glittenberg, C.(2007). Transplantation of the RPE in AMD. Prog. Retin. Eye Res.26, 516-554.
Buchholz, D. E., Hikita, S. T., Rowland, T. J., Friedrich, A. M., Hinman, C. R., Johnson, L. V. and Clegg, D. O.(2009). Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells27, 2427-2434.
Carr, A.-J., Vugler, A. A., Hikita, S. T., Lawrence, J. M., Gias, C., Chen, L. L., Buchholz, D. E., Ahmado, A., Semo, M., Smart, M. J. K. et al.(2009). Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS ONE4, e8152.
Carr, A.-J. F., Smart, M. J. K., Ramsden, C. M., Powner, M. B., da Cruz, L. and Coffey, P. J.(2013). Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci. doi: 10.1016/
j.tins.2013.03.006.
Chen, F. K., Uppal, G. S., MacLaren, R. E., Coffey, P. J., Rubin, G. S., Tufail, A., Aylward, G. W. and Da Cruz, L.(2009). Long-term visual and microperimetry outcomes following autologous retinal pigment epithelium choroid graft for neovascular age-related macular degeneration. Clin. Experiment. Ophthalmol.
37, 275-285.
Churchill, A. J., Carter, J. G., Lovell, H. C., Ramsden, C., Turner, S. J., Yeung, A., Escardo, J. and Atan, D.(2006). VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum. Mol. Genet.15, 2955-2961.
D’Cruz, P. M., Yasumura, D., Weir, J., Matthes, M. T., Abderrahim, H., LaVail, M. M. and Vollrath, D.(2000). Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet.9, 645-651. da Cruz, L., Chen, F. K., Ahmado, A., Greenwood, J. and Coffey, P.(2007). RPE
transplantation and its role in retinal disease. Prog. Retin. Eye Res.26, 598-635. de Jong, P. T. V. M.(2006). Age-related macular degeneration. N. Engl. J. Med.
355, 1474-1485.
Doi, A., Park, I.-H., Wen, B., Murakami, P., Aryee, M. J., Irizarry, R., Herb, B., Ladd-Acosta, C., Rho, J., Loewer, S. et al.(2009). Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat. Genet.41, 1350-1353.
Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okuda, S., Sekiguchi, K., Adachi, T. and Sasai, Y.(2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature472, 51-56.
Friedman, D. S., O’Colmain, B. J., Muñoz, B., Tomany, S. C., McCarty, C., de Jong, P. T. V. M., Nemesure, B., Mitchell, P., Kempen, J. and the Eye Diseases Prevalence Research Group(2004). Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol.122, 564-572. Fusaki, N., Ban, H., Nishiyama, A., Saeki, K. and Hasegawa, M.(2009).
Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci.85, 348-362.
Gal, A., Li, Y., Thompson, D. A., Weir, J., Orth, U., Jacobson, S. G., Apfelstedt-Sylla, E. and Vollrath, D.(2000). Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet.
26, 270-271.
D
E
V
E
LO
P
M
E
N
Garita-Hernández, M., Diaz-Corrales, F., Lukovic, D., Guede, I. G., Lloret, A. D., Sánchez, M. L. V., Massalini, S., Erceg, S. and Bhattacharya, S. S.(2013). Hypoxia increases the yield of photoreceptors differentiating from mouse embryonic stem cells and improves the modeling of retinogenesis in vitro.
Stem Cells31, 966-978.
Giansanti, F., Eandi, C. M. and Virgili, G.(2009). Macular translocation for neovascular age-related macular degeneration. Cochrane Database Syst. Rev.4, CD006928.
Gore, A., Li, Z., Fung, H.-L., Young, J. E., Agarwal, S., Antosiewicz-Bourget, J., Canto, I., Giorgetti, A., Israel, M. A., Kiskinis, E. et al.(2011). Somatic coding mutations in human induced pluripotent stem cells. Nature471, 63-67. Guha, P., Morgan, J. W., Mostoslavsky, G., Rodrigues, N. P. and Boyd, A. S.
(2013). Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell12, 407-412. Haines, J. L., Hauser, M. A., Schmidt, S., Scott, W. K., Olson, L. M., Gallins, P.,
Spencer, K. L., Kwan, S. Y., Noureddine, M., Gilbert, J. R. et al.(2005). Complement factor H variant increases the risk of age-related macular degeneration. Science308, 419-421.
Hamel, C. P., Jenkins, N. A., Gilbert, D. J., Copeland, N. G. and Redmond, T. M.(1994). The gene for the retinal pigment epithelium-specific protein RPE65 is localized to human 1p31 and mouse 3. Genomics20, 509-512.
Hartong, D. T., Berson, E. L. and Dryja, T. P.(2006). Retinitis pigmentosa. Lancet
368, 1795-1809.
Haruta, M., Sasai, Y., Kawasaki, H., Amemiya, K., Ooto, S., Kitada, M., Suemori, H., Nakatsuji, N., Ide, C., Honda, Y. et al.(2004). In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest. Ophthalmol. Vis. Sci.45, 1020-1025. Hirami, Y., Osakada, F., Takahashi, K., Okita, K., Yamanaka, S., Ikeda, H.,
Yoshimura, N. and Takahashi, M.(2009). Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci. Lett.458, 126-131. Humayun, M. S., de Juan, E., Jr, del Cerro, M., Dagnelie, G., Radner, W.,
Sadda, S. R. and del Cerro, C.(2000). Human neural retinal transplantation.
Invest. Ophthalmol. Vis. Sci.41, 3100-3106.
Idelson, M., Alper, R., Obolensky, A., Ben-Shushan, E., Hemo, I.,
Yachimovich-Cohen, N., Khaner, H., Smith, Y., Wiser, O., Gropp, M. et al. (2009). Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell5, 396-408.
Ikeda, H., Osakada, F., Watanabe, K., Mizuseki, K., Haraguchi, T., Miyoshi, H., Kamiya, D., Honda, Y., Sasai, N., Yoshimura, N. et al.(2005). Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc. Natl. Acad. Sci. USA102, 11331-11336.
Jager, R. D., Mieler, W. F. and Miller, J. W.(2008). Age-related macular degeneration. N. Engl. J. Med.358, 2606-2617.
Kim, D., Kim, C.-H., Moon, J.-I., Chung, Y.-G., Chang, M.-Y., Han, B.-S., Ko, S., Yang, E., Cha, K. Y., Lanza, R. et al.(2009). Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell4, 472-476.
Klimanskaya, I., Hipp, J., Rezai, K. A., West, M., Atala, A. and Lanza, R.(2004). Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells6, 217-245.
Kokkinaki, M., Sahibzada, N. and Golestaneh, N.(2011). Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells29, 825-835.
Kumar, A., Raj, M., Mochi, T. B., Mohanty, S., Seth, T. and Azad, R.(2012). Assessment of central retinal function after autologous bone marrow derived intravitreal stem cells injection in patients with retinitis pigmentosa using multifocal ERG: a pilot study. World J. Retina Vit.2, 5-13.
Lamba, D. A., Karl, M. O., Ware, C. B. and Reh, T. A.(2006). Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. USA103, 12769-12774.
Larijani, B., Esfahani, E. N., Amini, P., Nikbin, B., Alimoghaddam, K., Amiri, S., Malekzadeh, R., Yazdi, N. M., Ghodsi, M., Dowlati, Y. et al.(2012). Stem cell therapy in treatment of different diseases. Acta Med. Iran.50, 79-96. Li, Y., Reca, R. G., Atmaca-Sonmez, P., Ratajczak, M. Z., Ildstad, S. T., Kaplan,
H. J. and Enzmann, V.(2006). Retinal pigment epithelium damage enhances expression of chemoattractants and migration of bone marrow-derived stem cells. Invest. Ophthalmol. Vis. Sci.47, 1646-1652.
Li, M., Yu, A., Zhang, F., Dai, G., Cheng, H., Wang, X. and An, Y.(2012). Treatment of one case of cerebral palsy combined with posterior visual pathway injury using autologous bone marrow mesenchymal stem cells. J. Transl. Med.10, 100.
Liao, J. L., Yu, J., Huang, K., Hu, J., Diemer, T., Ma, Z., Dvash, T., Yang, X. J., Travis, G. H., Williams, D. S. et al.(2010). Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum. Mol. Genet.
19, 4229-4238.
Lu, B., Malcuit, C., Wang, S., Girman, S., Francis, P., Lemieux, L., Lanza, R. and Lund, R.(2009). Long-term safety and function of RPE from human
embryonic stem cells in preclinical models of macular degeneration. Stem Cells
27, 2126-2135.
Lu, B., Wang, S., Girman, S., McGill, T., Ragaglia, V. and Lund, R.(2010). Human adult bone marrow-derived somatic cells rescue vision in a rodent model of retinal degeneration. Exp. Eye Res.91, 449-455.
Lund, R. D., Wang, S., Klimanskaya, I., Holmes, T., Ramos-Kelsey, R., Lu, B., Girman, S., Bischoff, N., Sauvé, Y. and Lanza, R.(2006). Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells8, 189-199.
Lund, R. D., Wang, S., Lu, B., Girman, S., Holmes, T., Sauvé, Y., Messina, D. J., Harris, I. R., Kihm, A. J., Harmon, A. M. et al.(2007). Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells25, 602-611.
MacLaren, R. E., Pearson, R. A., MacNeil, A., Douglas, R. H., Salt, T. E., Akimoto, M., Swaroop, A., Sowden, J. C. and Ali, R. R.(2006). Retinal repair by transplantation of photoreceptor precursors. Nature444, 203-207. Martin, D. F., Maguire, M. G., Ying, G. S., Grunwald, J. E., Fine, S. L. and Jaffe,
G. J.(2011). Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med.364, 1897-1908.
McGill, T. J., Cottam, B., Lu, B., Wang, S., Girman, S., Tian, C., Huhn, S. L., Lund, R. D. and Capela, A.(2012). Transplantation of human central nervous system stem cells – neuroprotection in retinal degeneration. Eur. J. Neurosci.
35, 468-477.
Medeiros, N. E. and Curcio, C. A.(2001). Preservation of ganglion cell layer neurons in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci.42, 795-803.
Mellough, C. B., Sernagor, E., Moreno-Gimeno, I., Steel, D. H. W. and Lako, M.(2012). Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells30, 673-686.
Meyer, J. S., Shearer, R. L., Capowski, E. E., Wright, L. S., Wallace, K. A., McMillan, E. L., Zhang, S.-C. and Gamm, D. M.(2009). Modeling early retinal development with human embryonic and induced pluripotent stem cells.
Proc. Natl. Acad. Sci. USA106, 16698-16703.
Meyer, J. S., Howden, S. E., Wallace, K. A., Verhoeven, A. D., Wright, L. S., Capowski, E. E., Pinilla, I., Martin, J. M., Tian, S., Stewart, R. et al.(2011). Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells29, 1206-1218.
Nakano, T., Ando, S., Takata, N., Kawada, M., Muguruma, K., Sekiguchi, K., Saito, K., Yonemura, S., Eiraku, M. and Sasai, Y.(2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell
10, 771-785.
Osakada, F., Jin, Z.-B., Hirami, Y., Ikeda, H., Danjyo, T., Watanabe, K., Sasai, Y. and Takahashi, M.(2009). In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J. Cell Sci.122, 3169-3179. Otani, A., Dorrell, M. I., Kinder, K., Moreno, S. K., Nusinowitz, S., Banin, E.,
Heckenlively, J. and Friedlander, M.(2004). Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J. Clin. Invest.114, 765-774.
Owen, C. G., Jarrar, Z., Wormald, R., Cook, D. G., Fletcher, A. E. and Rudnicka, A. R.(2012). The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br. J. Ophthalmol.96, 752-756. Pappa, K. I. and Anagnou, N. P.(2009). Novel sources of fetal stem cells: where
do they fit on the developmental continuum? Regen. Med.4, 423-433. Park, S. S., Caballero, S., Bauer, G., Shibata, B., Roth, A., Fitzgerald, P. G.,
Forward, K. I., Zhou, P., McGee, J., Telander, D. G. et al.(2012). Long-term effects of intravitreal injection of GMP-grade bone-marrow-derived CD34+ cells in NOD-SCID mice with acute ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci.53, 986-994.
Pearson, R. A., Barber, A. C., Rizzi, M., Hippert, C., Xue, T., West, E. L., Duran, Y., Smith, A. J., Chuang, J. Z., Azam, S. A. et al.(2012). Restoration of vision after transplantation of photoreceptors. Nature485, 99-103.
Radtke, N. D., Aramant, R. B., Seiler, M. J., Petry, H. M. and Pidwell, D.(2004). Vision change after sheet transplant of fetal retina with retinal pigment epithelium to a patient with retinitis pigmentosa. Arch. Ophthalmol.122, 1159-1165.
Rosenfeld, P. J., Brown, D. M., Heier, J. S., Boyer, D. S., Kaiser, P. K., Chung, C. Y. and Kim, R. Y.(2006). Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med.355, 1419-1431.
Salero, E., Blenkinsop, T. A., Corneo, B., Harris, A., Rabin, D., Stern, J. H. and Temple, S.(2012). Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell10, 88-95. Sauvé, Y., Girman, S. V., Wang, S., Keegan, D. J. and Lund, R. D.(2002).
Preservation of visual responsiveness in the superior colliculus of RCS rats after retinal pigment epithelium cell transplantation. Neuroscience114, 389-401. Schwartz, S. D., Hubschman, J.-P., Heilwell, G., Franco-Cardenas, V., Pan, C.
K., Ostrick, R. M., Mickunas, E., Gay, R., Klimanskaya, I. and Lanza, R. (2012). Embryonic stem cell trials for macular degeneration: a preliminary
report. Lancet379, 713-720.
D
E
V
E
LO
P
M
E
N
Singhal, S., Bhatia, B., Jayaram, H., Becker, S., Jones, M. F., Cottrill, P. B., Khaw, P. T., Salt, T. E. and Limb, G. A.(2012). Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl. Med.1, 188-199.
Siqueira, R. C., Messias, A., Voltarelli, J. C., Scott, I. U. and Jorge, R.(2011). Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial. Retina31, 1207-1214. Sparrow, J. R., Hicks, D. and Hamel, C. P.(2010). The retinal pigment
epithelium in health and disease. Curr. Mol. Med.10, 802-823.
Strauss, O.(2005). The retinal pigment epithelium in visual function. Physiol. Rev.
85, 845-881.
Takahashi, K. and Yamanaka, S.(2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell
126, 663-676.
Tezel, T. H., Del Priore, L. V. and Kaplan, H. J.(2004). Reengineering of aged Bruch’s membrane to enhance retinal pigment epithelium repopulation.
Invest. Ophthalmol. Vis. Sci.45, 3337-3348.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S. and Jones, J. M.(1998). Embryonic stem cell lines derived from human blastocysts. Science282, 1145-1147.
Tsukahara, I., Ninomiya, S., Castellarin, A., Yagi, F., Sugino, I. K. and Zarbin, M. A.(2002). Early attachment of uncultured retinal pigment epithelium from aged donors onto Bruch’s membrane explants. Exp. Eye Res.74, 255-266. van Zeeburg, E. J. T., Maaijwee, K. J. M., Missotten, T. O. A. R., Heimann, H.
and van Meurs, J. C.(2012). A free retinal pigment epithelium–choroid graft in patients with exudative age-related macular degeneration: results up to 7 years. Am. J. Ophthalmol.153, 120-127.e2.
Vugler, A., Carr, A.-J., Lawrence, J., Chen, L. L., Burrell, K., Wright, A., Lundh, P., Semo, M., Ahmado, A., Gias, C. et al.(2008). Elucidating the
phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp. Neurol.214, 347-361.
Walia, S. and Fishman, G. A.(2009). Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet.30, 63-68.
Warren, L., Manos, P. D., Ahfeldt, T., Loh, Y.-H., Li, H., Lau, F., Ebina, W., Mandal, P. K., Smith, Z. D., Meissner, A. et al.(2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell7, 618-630.
Yang, Z., Chen, Y., Lillo, C., Chien, J., Yu, Z., Michaelides, M., Klein, M., Howes, K. A., Li, Y., Kaminoh, Y. et al.(2008). Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J. Clin. Invest.118, 2908-2916.
Ying, Q.-L., Nichols, J., Evans, E. P. and Smith, A. G.(2002). Changing potency by spontaneous fusion. Nature416, 545-548.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R. et al.(2007). Induced pluripotent stem cell lines derived from human somatic cells. Science
318, 1917-1920.
Zhang, K., Kniazeva, M., Han, M., Li, W., Yu, Z., Yang, Z., Li, Y., Metzker, M. L., Allikmets, R., Zack, D. J. et al.(2001). A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat. Genet.
27, 151-152.
Zhao, T., Zhang, Z.-N., Rong, Z. and Xu, Y.(2011). Immunogenicity of induced pluripotent stem cells. Nature474, 212-215.
Zhu, D., Deng, X., Spee, C., Sonoda, S., Hsieh, C. L., Barron, E., Pera, M. and Hinton, D. R.(2011). Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Invest. Ophthalmol. Vis. Sci.52, 1573-1585.
Zimmermann, A., Preynat-Seauve, O., Tiercy, J.-M., Krause, K.-H. and Villard, J.(2012). Haplotype-based banking of human pluripotent stem cells for transplantation: potential and limitations. Stem Cells Dev.21, 2364-2373.