Chapter 10 Micronutrients II: Trace Elements
Chapter 10 Micronutrients II: Trace Elements ... 357
Individual trace elements. ... 362
Zinc ... 362 Copper... 365 Selenium... 367 Manganese ... 367 Chromium ... 368 Molybdenum ... 369 Fluoride ... 369 Iodine ... 370
Figure 10.1 Acrodermatitis enteropathica showing typical distribution of skin rash. ... 364
Figure 10.2 Endemic Goitre... 370
Figure 10.3 Goitre ... 370
Figure 10.4 Cretinism ... 371
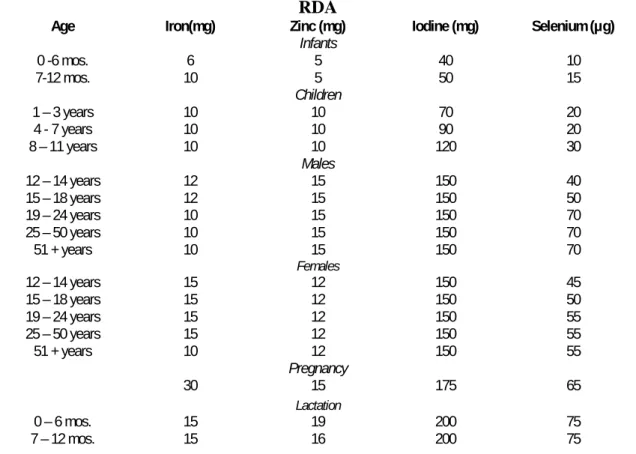
Table 10.1 Recommended daily Allowances for Iron, Zinc, Iodine and Selenium ... 358
Table 10.2 Safe and adequate intake for five minerals ... 359
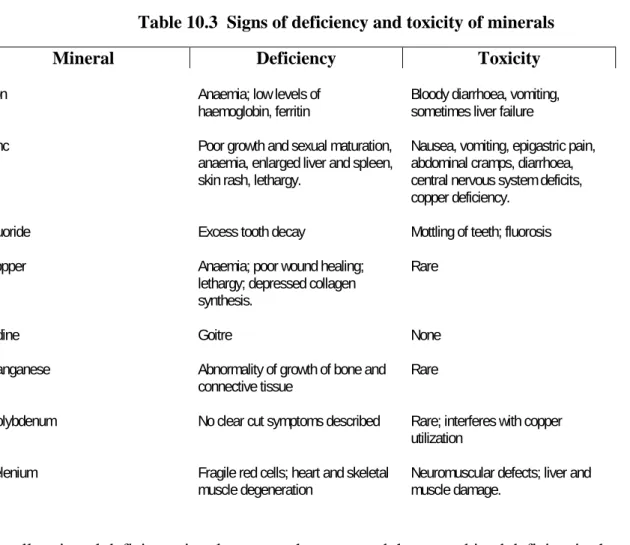
Table 10.3 Signs of deficiency and toxicity of minerals... 359
Table 10.4 Functions served by trace minerals... 360
Table 10.5 Etiology of trace element deficiency ... 361
Table 10.6 Food sources of zinc ... 363
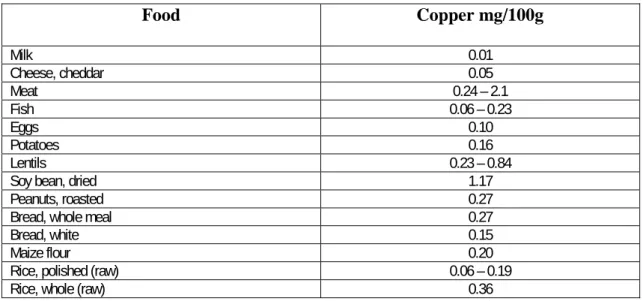
Table 10.7 Food sources of copper ... 365
Table 10.8 Food sources of Magnesium ... 368
357 1 1 1 1 1 1 1 1 1 1 1 1
The human body needs a number of minerals in trace (milligram) quantities. These include iron, copper and zinc. Other minerals are required in ultra trace (microgram) amounts. These are
chromium, manganese, fluoride, iodide, cobalt, selenium, silicon, arsenic, boron, and vanadium. Of the 18 micro minerals thought to be essential only 4 have been studied in detail and the
recommended daily allowance has been worked out. (See table 10.1)
Table 10.1 Recommended daily Allowances for Iron, Zinc, Iodine and Selenium RDA
Age Iron(mg) Zinc (mg) Iodine (mg) Selenium (µg)
Infants 0 -6 mos. 6 5 40 10 7-12 mos. 10 5 50 15 Children 1 – 3 years 10 10 70 20 4 - 7 years 10 10 90 20 8 – 11 years 10 10 120 30 Males 12 – 14 years 12 15 150 40 15 – 18 years 12 15 150 50 19 – 24 years 10 15 150 70 25 – 50 years 10 15 150 70 51 + years 10 15 150 70 Females 12 – 14 years 15 12 150 45 15 – 18 years 15 12 150 50 19 – 24 years 15 12 150 55 25 – 50 years 15 12 150 55 51 + years 10 12 150 55 Pregnancy 30 15 175 65 Lactation 0 – 6 mos. 15 19 200 75 7 – 12 mos. 15 16 200 75 358 1 1 1 1 1 1 1 1 1 1 1 1
Additional five minerals have been studied sufficiently so as to make recommendations for intakes judged safe and adequate. (See table 10.2)
Table 10.2 Safe and adequate intake for five minerals
Mineral Intake Copper 1.5 – 3.0 mg/day Fluoride 1.4 – 4.0 mg/day Manganese 2.0 – 5.0 mg/day Chromium 50 -200 µg/day Molybdenum 75 – 200 µg/day
Excess exposure to certain minerals can invoke the generation of free radicals. Free radicals can damage cell membranes altering their function, and can target several intracellular constituents like enzymes, transporters, signalling systems and even DNA. Table 10.3 gives a general description of signs of deficiency and of toxicity for the more important minerals.
Table 10.3 Signs of deficiency and toxicity of minerals
Mineral Deficiency Toxicity
Iron Anaemia; low levels of
haemoglobin, ferritin Bloody diarrhoea, vomiting, sometimes liver failure
Zinc Poor growth and sexual maturation,
anaemia, enlarged liver and spleen, skin rash, lethargy.
Nausea, vomiting, epigastric pain, abdominal cramps, diarrhoea, central nervous system deficits, copper deficiency.
Fluoride Excess tooth decay Mottling of teeth; fluorosis
Copper Anaemia; poor wound healing;
lethargy; depressed collagen synthesis.
Rare
Iodine Goitre None
Manganese Abnormality of growth of bone and
connective tissue Rare
Molybdenum No clear cut symptoms described Rare; interferes with copper utilization
Selenium Fragile red cells; heart and skeletal
muscle degeneration Neuromuscular defects; liver and muscle damage.
Usually mineral deficiency involves more than one, and these combined deficiencies have cumulative effect on health.
359 1 1 1 1 1 1 1 1 1 1 1 1
Absorption. Few enter the enterocyte by passive transfer. Several are carried into the body by proteins to which the minerals are loosely attached. Iron, zinc and copper are imported via such transporter proteins. The absorption process is dependent upon binding capacity of the transporter protein; solubility; the mixture of other minerals present in the gut content; and the presence of phytates which bind specific minerals and thereby change the mineral content presented to the enterocytes. Iodide and fluoride enter by way of anion-cat ion exchange mechanisms. All these factors determine the bioavailability of the minerals. In addition physiological factors like age, hormonal status and health status also influence absorption and subsequent use.
Bioavailability. Bioavailability has been defined as the percentage of the consumed mineral that enters via the enterocyte and is used for its intended purpose. Bioavailability includes not only how much of a consumed mineral enters the body but also how much of it is retained and available for use. For example, iron in meat has a greater bioavailability than iron from spinach, because the haem form of iron in meat is absorbed better whereas in spinach it is bound to oxalate and is in the ferric form.
Many of the minerals can be chelated by organic materials, and many of the chelating agents can chelate several minerals. This is important to know from the clinical point of view. For example EDTA when used as a chelating agent in lead overload also removes calcium and magnesium. Functions of minerals. They serve a variety of functions. Some have only one function e.g. cobalt in vitamin B12 and iodine in thyroxine. But others serve multiple functions ranging from acting as
cofactors in enzyme reactions to stabilizing and contributing to the hardness of bone. Metal ions occur almost always bound to particular proteins where they often play a crucial part in maintaining the protein’s three dimensional structure. If the protein happens to be an enzyme then the metal may be needed for catalytic activity. Several of the minerals influence the expression of genes encoding one or more proteins by regulating the transcription and translation of the gene. In this respect zinc has been studied the most. Copper and iron are also similarly involved. The various functions served in the body by trace elements are summarised in Table 10.4, and further discussed in detail later under individual elements.
Table 10.4 Functions served by trace minerals. Mineral Function
Iron Essential component of haemoglobin and the cytochromes. Serves in the expression of genes for receptors of ferritin, trans ferritin and metallothioneins.
Zinc Cofactor in more than 100 enzymatic reactions. Essential component of nuclear DNA binding proteins; serves in the expression of genes for metallothioneins.
Copper Essential cofactor in several reactions concerning iron use, collagen synthesis, and suppression of free radicals. Serves in the expression of genes for several enzymes. Cobalt Essential in the structure of vitamin B 12.
Iodine Essential for synthesis of thyroid hormone. Manganese Essential cofactor in many enzymatic reactions.
Selenium Essential for enzyme reactions for glutathione and thyroxine. Fluorine Increases hardness of teeth; prevents caries.
360 1 1 1 1 1 1 1 1 1 1 1 1
Many of the functions described in table 12.4 are vital metabolic functions. Deficiency of trace minerals can produce a variety of diseases. It is the study of such diseases that has helped to provide an understanding of the role of trace minerals in body metabolism. Iron, copper, zinc, molybdenum, selenium and manganese have been identified as constituents of specific enzyme systems. Iron and copper can exist in two oxidation states. Ferrous can be oxidized to ferric and ferric reduced back to ferrous. A number of enzymes make use of this property of iron and copper. During the oxidation of glucose iron bound to mitochondrion protein cytochrome is alternately oxidised to ferric and
reduced to ferrous state. Another cytochrome utilizes the oxidation and reduction of its copper component in a similar way. Certain trace elements are of fundamental importance in the structure or metabolic activity of important compound including haemoglobin (iron), vitamin B12 (cobalt),
and nucleic acids (multiple trace elements). The role of iodine in thyroxine and triiodothyronine and of chromium in facilitating the action of insulin is examples of the role of trace elements in the activity of certain hormones. The dependence of many vital metabolic processes on trace elements confers upon them a physiologic importance analogous to that of vitamins.
Aetiology of trace elements deficiency. A number of factors play a role in causing a deficiency of trace elements. These are summarised in table 10.5 and further discussed under individual elements later.
Table 10.5 Etiology of trace element deficiency
Aetiology Trace element affected
Inadequate intake
Increased risk during infancy and pregnancy Iron, Zinc
Losses during food processing including manufacture of “humanized
milk” formulae Zinc, Chromium, Copper, Selenium
Poor bioavailability Iron, Zinc
Local deficiencies in the soil Iodine, Selenium
As part of protein-energy malnutrition Zinc, Copper, Chromium Synthetic diets including intravenous feeding Zinc, Copper, Chromium, Selenium,
Molybdenum
Other circumstances
Prematurity Iron, Copper, Zinc
Intestinal disease causing impaired absorption or excessive loss of trace elements
Zinc, Copper Chromium Inborn errors of metabolism of trace elements Zinc, Copper, Molybdenum
361 1 1 1 1 1 1 1 1 1 1 1 1
Diseases that cause intestinal malabsorption of fat and other nutrients may also cause trace element deficiencies.
Some elements especially copper and iron are stored by the foetus during the last trimester of pregnancy. These stores are diminished or even lacking in the infant born preterm. Immaturity of intestinal absorption mechanisms and relatively high urinary losses compound the problem in preterm infants. At the same time rapid growth in the early postnatal period impose high nutritional requirements. Nutritional requirements may also be high during periods of rapid growth in the term infant as well as during pregnancy and lactation.
Trace element deficiencies occur in defined geographical areas owing to deficiencies in soils, plants or water. For example, iodine deficiency is often found in mountainous regions and in river delta areas. Diets deficient in other nutrients may also contain inadequate quantities of trace elements. Losses occur during food processing. For example, it has been estimated that the milling of grain removes more than three-quarters of the zinc and manganese, and also substantial amounts of copper and molybdenum. Refining of sugar also causes losses of trace elements. “Humanizing” of cow’s milk can inadvertently cause trace mineral deficiencies. There is much variation to be found in trace element concentration in different formulas on a world-wide basis. Variation in
bioavailability is especially important for the infant because of dependence on just one food item, milk, for the first four to six months of life. If the feeds are deficient in any one trace element there is no chance for the infant to make good the deficit by a favourable supply from other food item. In this respect human milk has an important advantage with regard to the bioavailability of iron and zinc, and this may also be true for other trace elements.
Individual trace elements.
Zinc
The requirement for zinc is 1 mg/day in infancy; 10 mg/day between 1 and 10 years of age, and 15 mg/day in adults. It is not generally appreciated that as much zinc as iron is required in the diet. There is no body store for zinc unlike iron. All the zinc is locked in bone or protein which explains the rapidity of onset of symptoms on a deficient diet. In this respect zinc resembles essential amino acids. This similarity as well as the non-specific nature of zinc deficiency signs suggests that zinc deficiency causes a block in protein and nucleic acid synthesis. The immune system, the skin and the gastro-intestinal tract are the tissues of the body with the highest rate of protein synthesis, and they are the main targets for deficiency signs to appear.
362 1 1 1 1 1 1 1 1 1 1 1 1
The major food sources of zinc are listed in Table 10.6.
Table 10.6 Food sources of zinc
Food Zinc (mg/100 g) Milk 0.4 Cheese, cheddar 2.3 Meat 1.5 – 8.7 Fish 0.5 Eggs 1.3 Potatoes 0.3 Lentils 0.77 – 2.8
Soy bean, dried 3.0
Peanuts, roasted 3.2 Ginger 4.5 Bread, wholemeal 2.0 Bread, white 0.8 Chapatti 1.0 Maize flour 2.5
Rice, polished (raw) 1.3
Rice, Whole (raw) 1.4
In animal food sources the fat content becomes a major determinant of the zinc content because fat tissue contains much less zinc than muscle tissue. In general red meat has more zinc than white meat, and fish has less zinc than meat. The zinc content of colostrum is high. Over the first few weeks after delivery the concentration in breast milk falls but is usually sufficient to meet the needs of the term infant.
Cereals are a major source of energy and zinc in large parts of the world. The amount depends on the refinement of the flour. Zinc is mainly located in the outer layers of the grain so that in highly refined flour a major portion of zinc as well as other minerals gets removed. The content of zinc in vegetables is dependent on the variety, class and location. Up to 10-fold variation in the zinc content of wheat has been reported depending on the botanical variety. Green leafy vegetables and fruit are only modest sources of zinc because of their high water content. A dietary factor that can impair zinc absorption is phytate present in whole grain cereal, legumes and other vegetables. Absorption of zinc occurs by a saturable mechanism that is carrier mediated. A number of ligands facilitate uptake by the mucosal cells. Zinc attached to transferrin travels from the mucosal
enterocytes to the liver in the portal circulation. In the systemic circulation approximately two-thirds of plasma zinc is loosely bound to albumin and the remainder to globulin. In the tissues highest known concentrations of zinc occur in the eye, bone and hair, and to a lesser extent in muscle and kidney.
The primary route of excretion is by way of the gastro-intestinal tract with desquamation of mucosal cells.
Physiological role. Zinc is involved in nucleic acid synthesis, protein metabolism, carbohydrate metabolism, bone metabolism, oxygen transport and protection against free radical damage. Such a large variety of functions is possible because zinc is a constituent of several hundred metallo-enzymes. In some of these enzymes zinc is present at the active site; in others and in some non-enzyme proteins the function of zinc is structural. Zinc helps to stabilise the structures of DNA,
363 1 1 1 1 1 1 1 1 1 1 1 1
RNA, and ribosomes. It is involved in normal chromatin restructuring, and in gene expression. Zinc also seems to have an important role in the structure and function of membranes. It also plays an important role in the immune system especially T-cell function.
A meta analysis of 33 prospective intervention studies of zinc supplementation and its effects on children’s growth in many countries showed that zinc supplementation alone had a significant effect on linear growth and gain in weight. Zinc supplementation has been shown to reduce the incidence and duration of acute and persistent diarrhoea as well as acute respiratory infection in children. Signs of zinc deficiency. Decrease in growth velocity or total arrest of growth is a consistent and early result of zinc deficiency in infants, children and adolescents. In acute zinc deficiency states e.g. acrodermatitis enteropathica (See description below and fig.10.1) there is an abrupt cessation of weight gain and appearance of skin rash. It presents a few weeks following birth after foetal stores are exhausted.
Figure 10.1 Acrodermatitis enteropathica showing typical distribution of skin rash.
Acrodermatitis enteropathica is an autosomal recessively inherited disorder in which there is a partial block in the intestinal absorption of zinc. It used to be a severe, progressive and even fatal condition before the benefits of zinc therapy were realised.
Factor or factors in human milk help to ameliorate the clinical and biochemical abnormalities. Catch-up growth occurs when zinc supplements are administered. The skin lesions have a characteristic distribution, primarily on the extremities and adjacent to body orifices. Secondary infection of the vesicles is common and difficult to heal. Frequent bacterial and monilial infections due to abnormalities of the immune system are common.
Many of the features of zinc deficiency like, for example, growth retardation, poor wound healing, abnormalities in the immune system, are attributable to disturbance in nucleic acid metabolism and protein synthesis. Sodium transport across cell membrane is affected by zinc deficiency which explains the diarrhoea characteristic of zinc deficiency.
Etiological factors in zinc deficiency. Dietary zinc deficiency is rare and occurs only in
exceptional circumstances in the case of subjects on synthetic diets, or because of factors in the diet that interfere with bioavailability like high levels of phytate and fibre together with low levels of animal protein. 364 1 1 1 1 1 1 1 1 1 1 1 1
Bioavailability of zinc from human milk is much better than with cow’s milk. “Humanized” cow’s milk formulae need zinc supplementation, but a wide variation in zinc content of infant formulae has been reported.
In diseases of the gastro-intestinal system malabsorption of zinc or excessive losses occur. Fat malabsorption or inflammatory bowel disease can impair zinc absorption as is the case with regional ileitis, celiac disease, cystic fibrosis and persistent diarrhoea. In protein-energy
malnutrition there is often global deficiency of nutrients including micronutrients. Deficiency of zinc is contributes to reduced immunocompetence and increased morbidity from infectious diseases including diarrhoea. During diarrhoea further losses of zinc occur in stools in these children. In patients on total parenteral nutrition zinc deficiency is precipitated by failure to add zinc supplements to intravenous feeding.
Treatment of zinc deficiency. Most cases of zinc deficiency can be treated with zinc 1 mg/kg body weight per day to a maximum of 20 mg per day of zinc. (1 mg of elemental zinc = 4.4 mg of zinc sulphate; 7 mg of zinc gluconate, and 2.8 mg of zinc acetate).
Copper
The copper content of soil determines the amount in foodstuff. Dairy products are generally poor source of copper. Whole grain cereals, organ meats, shell fish, nuts, dried legumes are good sources of copper. (See Table 10.7). Human milk contains 200 – 600 µg/litre. Absorption from breast milk is high because of presence of ligands that facilitate absorption. Cow’s milk contains 150 µg/litre. Infant formulae show a wide variation world-wide.
Table 10.7 Food sources of copper
Food Copper mg/100g Milk 0.01 Cheese, cheddar 0.05 Meat 0.24 – 2.1 Fish 0.06 – 0.23 Eggs 0.10 Potatoes 0.16 Lentils 0.23 – 0.84
Soy bean, dried 1.17
Peanuts, roasted 0.27
Bread, whole meal 0.27
Bread, white 0.15
Maize flour 0.20
Rice, polished (raw) 0.06 – 0.19
Rice, whole (raw) 0.36
365 1 1 1 1 1 1 1 1 1 1 1 1
Some 40% of ingested copper is absorbed in the stomach and small intestine; the proportion absorbed depending upon the dietary form and other constituents of diet like calcium, iron, or zinc which interfere with absorption. Absorbed copper travels bound to albumin in the portal circulation to the liver. Ceruloplasmin is formed in the liver and most copper circulates in the blood bound to ceruloplasmin. Very little of this copper is exchanged daily, so ceruloplasmin cannot play a major part in absorption and transport. Remaining copper is bound to albumin and is believed to constitute true transport copper.
Copper forms part of a number of metallo-enzymes involved in the cytochromes chain in
mitochondria, in the synthesis of proteins of collagen tissue in the skeleton and blood vessels, and in the synthesis of neurotransmitters and neuropeptides. In particular cytochrome C which is responsible for the production of most of the energy of metabolism is markedly reduced in the brains of experimental animals. In the adult 40 per cent of the 80mg of copper is located in muscle, 15 per cent in liver, 10 per cent in the brain, and 6 per cent in blood. The term infant is born with substantial stores in the liver. The normal newborn’s hepatic concentration of copper is ten times higher than that in adults. Laying down of stores of copper occurs during the last trimester of pregnancy. In the pre-term infant liver stores are smaller and may develop deficiency after about 2 months of age.
Copper deficiency disease is rare except in specific groups such as very low birth weight infant, in children treated for protein energy malnutrition with a milk based diet low in copper for a long period, in children with protracted diarrhoea, and during parenteral feeding.
Copper deficiency leads to intracellular abnormality of iron metabolism because of defective iron transport causing inefficient production of heme, and a hypochromic anaemia not responding to iron. In pre-term infants copper deficiency may present as severe osteoporosis with cupping and flaring of the bone ends, periosteal reaction and sub-metaphyseal fractures. Skin rash similar to seborrhoeic dermatitis, failure to thrive, psychomotor retardation and heptosplenomegaly are other signs of copper deficiency.
Response to copper 2 – 3 mg daily as copper sulphate 1% solution is rapid.
Two conditions due to abnormalities of copper metabolism have been described. Wilson’s disease (hepatolenticular degeneration) is an autosomal recessive condition in which excessive
accumulation of copper occurs in tissues. Accumulation in erythrocytes, liver, brain and kidneys accounts for most of the symptoms. In erythrocytes it leads to acute haemolytic anaemia. Hepatic insufficiency is followed by cirrhosis and liver failure. Accumulation of copper in nerve cells causes tremors, choreo-athetoid movements and eventually dementia. Menke’s steely hair syndrome is a severe and eventually fatal involvement of the central nervous system due to disorders of copper metabolism in brain cells.
366 1 1 1 1 1 1 1 1 1 1 1 1
A third condition, thought to be very likely due to liver damage by excessive ingestion of copper has been described from India. IndianChildhood Cirrhosis is a disease of infants and young children described from India. Liver biopsy in advance stages shows widespread damage to liver cells causing irreversible damage. Using special staining technique of biopsied liver tissue several authors have described presence of dark brown orcein-staining granules which represent copper associated protein. It is believed that milk stored overnight in copper vessels, a common custom in India, leaches out copper from the container. Over a period of time chronic poisoning with copper occurs. Indian Childhood Cirrhosis has not been reported in Indians living abroad in Africa, Europe and North America showing that it is peculiar to the way of life of Indian customs practiced in the home country.
Selenium
Selenium levels in soil vary widely which is reflected in the crops that grow on the soil. In animals disorders due to excess or deficiency have been recognised. In humans one condition called Keshan disease has been reported affecting otherwise normal individuals in a large part of China extending from northeast to the southwest of the country. The disease is thought to be associated with
consumption of the local food staple in which selenium content is very low. Keshan disease affects largely children and women in the childbearing age. It is an endemic form of cardiomyopathy in which there are multiple foci of myocardial necrosis. Its presentation may be acute with heart failure, arrhythmia and cardiogenic shock, or chronic with cardiomegaly with or without congestive failure. The electrocardiogram shows low voltage and a bundle-branch or complete atrio-ventricular block. Selenium supplementation (0.5 – 1.0 mg given weekly) brings down the incidence.
Biochemistry. Selenium has an important role as an essential component of glutathione peroxidase in which it provides the active site. This enzyme utilizes two molecules of reduced glutathione to reduce hydrogen peroxide and convert it to two molecules of water. It also catalyzes the reduction of fatty acid hydro peroxides to hydroxy acids in the tissues and thus helps to protect the lipids of cell membranes fro peroxidation. Selenium is thus important for maintaining membrane stability and for controlling free radical damage. Glutathione peroxidase is present in a wide variety of tissues and accounts for 90 per cent of the selenium in erythrocytes.
Absorption of selenium depends on the chemical form, the organic form being better absorbed than the inorganic. Intestinal absorption can be as much as 80 per cent. Principal route of excretion is by way of the kidney. Highest tissue concentrations are found in the liver, tooth enamel, and nails. Dietary requirements and the food sources. Human milk contains 15 – 20 µg/litre. Thus the intake in a young breast fed infant is about 3 µg/kg body weight per day. The concentration in foods varies with geographical region and soil content. Sea food (0.5 µg/g), kidney, liver, meat (approximately 0.2 µg/g), and whole grains are good sources. Vegetables and fruits provide little selenium.
Manganese
Manganese is required for the synthesis of mucopolysaccharides through the enzymes polymerase and galacto-transferase. Only about 3 per cent of an ingested dose is absorbed.
Nuts and unrefined grain are rich sources of manganese. Vegetables and fruits contain moderate amounts. Animal products and sea foods are relatively low in manganese.
367 1 1 1 1 1 1 1 1 1 1 1 1
Magnesium
Magnesium is an intracellular ion which acts as a cofactor in oxidative phosphorylation in the synthesis or utilization of ATP (e.g. in muscle contraction). Many of the adhesion molecules i.e. proteins involved in direct cell-cell contact have magnesium located at the binding site of the molecule. Its plasma concentration relative to calcium affects nerve transmission and muscular contraction. The concentration of substances like fatty acids, phytate, and phosphorous in the gut contents affects magnesium absorption. Its absorptive pathway is common with calcium.
Food sources. Magnesium is widely present in many foods especially whole grain cereals and green vegetables. (See Table 10.8). Refining the flour, rice and sugar causes considerable loss of
magnesium. Breast milk contains about 35mg/l. Its absorption from cow’s milk is also good.
Table 10.8 Food sources of Magnesium
Food Magnesium (mg/100 g)
Milk, cow’s whole 11
Cheese, cheddar 25 Eggs 12 Meat 21 - 24 Fish 25 – 50 Cabbage 4 Potatoes 14
Peanuts, dry roasted 190
Bread, white 24
Bread, wholemeal 76
Chapatti 37
Chromium
Chromium acts as a cofactor for insulin by facilitating attachment of insulin to receptors on the surface of the target cell as well as within the cell. It forms a complex compound made up of chromium-amino acid-nicotinic acid which is referred to as the glucose tolerance factor. It is believed that chromium crosses the placenta only as the glucose tolerance factor.
A large proportion of chromium is absorbed from organic chromium complexes present in food. Major excretory route is the kidney. The urine contains 80 per cent of excreted chromium. Food sources of chromium are yeast, liver, and kidney. Small quantities are present in fish, vegetables, and fruits. Major losses of chromium occur during milling of wheat and refining of sugar. 368 1 1 1 1 1 1 1 1 1 1 1 1
Molybdenum
It acts as an agent for electron transfer. Xanthine oxidase is molybdenum containing metallo enzyme which has a role in purine metabolism. Xanthine dehydrogenase is nearly identical in structure and function to Xanthine oxidase.
Food sources. Drinking water is a minor source (0.20 µg/litre except where mining effluents find their way into the water supply). Foods with highest levels of molybdenum are dried legumes, grains, cereal products and organ meats.
Fluoride
Adequate intake is obtained in geographical areas where the water supply is fluoridated to a level of 1mg/litre. Daily intake of breastfed infants is about 5 µg in low fluoride areas and 8 µg in high fluoride areas.
Main source of fluorine is the drinking water which is considered ideal if it contains 1 part per million. Sea fish and tea are other rich sources.
Accumulation of fluoride occurs in teeth and bones after prolonged high intake of fluorine. The condition is called fluorosis. Teeth are more commonly affected in the form of chalky white patches distributed irregularly over the surface of the enamel. Teeth mottling is proportional to fluoride content of the water supply, being mild at a level of 4 parts per million, and moderate to severe at 14 parts per million. The enamel on the affected teeth gets weakened and erodes giving rise to characteristic pitting. The changes in the bones are in the form of osteosclerosis and exostosis.
369 1 1 1 1 1 1 1 1 1 1 1 1
Iodine
Iodine deficiency occurs in certain mountainous regions and areas prone to recurrent flooding. (See Fig.10.2)
Figure 10.2 Endemic Goitre
Iodine is necessary for the synthesis of the thyroid hormone. Enlargement of the thyroid gland occurs when iodine intake is less than 15 µg/day. Most goiterous persons are clinically euthyroid, and the only person is cosmetic. Of the two types of thyroid hormones circulating in the blood of such persons T4 decreases but T3 remains normal. The levels of thyroid stimulating hormone are raised causing enlargement of the thyroid gland, hence the goitre. (See Fig. 10.3)
Figure 10.3 Goitre 370 1 1 1 1 1 1 1 1 1 1 1 1
Maternal iodine deficiency during pregnancy gives rise to cretinism, of which two types have been described, “neurologic” and “myxoedematous”. The neurologic type prevails in most regions. In goitre endemic areas 1 to 10 per cent of newborns show effects of neonatal hypothyroidism compared with only 0.025 per cent in iodine sufficient regions. Iodine deficiency during the foetal and early neonatal period adversely affects the growing brain. The parts most affected are the cerebral neocortex, the cochlea, and the basal ganglia all of which grow rapidly during the second trimester and are vulnerable to iodine deficiency. This explains the combination of mental
deficiency, deaf-mutism and motor rigidity found in cretinism. (See Fig. 10.4)
Figure 10.4 Cretinism
Supplementation with iodine through salt has been used as the main plank of the strategy of
prevention. Even then marginal areas are likely to be missed out. Health education of the public and vigilance in the enforcement of using iodized salt are additional aspects of prevention.
371 1 1 1 1 1 1 1 1 1 1 1 1
BLANK PAGE 372 1 1 1 1 1 1 1 1 1 1 1 1