Integrated and biological control of parasites in organic
and conventional production systems
S.M. Thamsborg
a,b,∗, A. Roepstorff
b, M. Larsen
baOrganic Animal Health and Production, Department of Animal Science and Animal Health, Royal Veterinary
and Agricultural University, 2 Grønnegårdsvej, DK-1870 Frederiksberg, Denmark
bDanish Centre for Experimental Parasitology, Royal Veterinary and Agricultural University, Copenhagen,
Denmark
Abstract
Organic and other non-intensive animal production systems are of growing importance in several countries worldwide. In contrast to conventional farms, parasite control on organic farms is affected by several of the prescribed changes in management e.g. access to the outdoors in the summer and in most countries, a ban on preventive medication, including use of anti-parasiticides. Organic an-imal production relies heavily on grazing, and pasture or soil related parasites are thus of major importance. Several studies in northern temperate climate have indicated that outdoor production of pigs, primarily sows, and laying hens results in heavier and more prevalent helminth infections compared to conventional intensive production under indoor conditions. In organic dairy cattle, parasitic gastroenteritis in heifers may be more prevalent. In a short to medium term perspective, integrated control may combine grazing management with biological control using nematophagous micro-fungi, selected crops like tanniferous plants and on conventional farms, limited use of anti-parasiticides. At present, the non-chemotherapeutic control of pasture related infections is based mainly on grazing management strategies. Preventive strategies, where young, previously unex-posed stock, are turned out on parasite-free pastures, can be used for grazing first season dairy heifers and in all-in-all-out poultry production. Evasive strategies aim at avoiding disease produc-ing infections of a contaminated area by movproduc-ing to a clean area and may be relevant for ruminants and pigs. In cattle, effective control of nematodes can be achieved by repeated moves of the herd or alternate grazing with other species. High stocking rates seem to be an important risk factor. In pig production, the effect of paddock rotation on parasite infections is largely unknown and studies are warranted. Control of nematodes by larvae-trapping fungi, or perhaps in the future by egg-destroying fungi, looks promising for ruminants and certain monogastric animals but delivery systems and practical dosing regimes integrated with grazing management have to be developed. In conclusion, good prospects are expected for acceptable parasite control without a heavy reliance on anti-parasiticides through integration of the above mentioned procedures but future studies are needed to confirm their efficacy under practical farming conditions. ©1999 Elsevier Science B.V. All rights reserved.
∗Corresponding author. Tel.: +45 3528 3028; fax: +45 3528 3055; e-mail: smt@kvl.dk 0304-4017/99/$ – see front matter ©1999 Elsevier Science B.V. All rights reserved. PII: S 0 3 0 4 - 4 0 1 7 ( 9 9 ) 0 0 0 3 5 - 7
Table 1
Legislative rules with relevance to parasite control in certified organic farms in Denmark (Anon., 1998) Grazing at least 150 days in summer or access to outdoor yards
Preventive use of medicine, e.g. anthelmintics and coccidiostats, is prohibited Specific diagnosis by veterinarian before treatment
Withdrawal time following medical treatment is three times the statutory time Preferable oral anthelmintics
Daily access to roughage (or grazing)
1. Setting the scene: introduction and basic concepts
Organic agriculture has expanded in many European countries over the last ten years. This reflects increasing concern over environmental issues in intensive agriculture, in particular, problems with the use of pesticides and commercial fertilizers. There is also an increasing market for products perceived as healthy and a concern for animal welfare. This development has been driven largely by consumer demand and visionary farmers, and later supported by financial premiums or direct subsidies. Only in recent years has organic farming been supported by the research establishment.
Organic farming encompasses a concept of a more sustainable approach to agricultural production. It aims at maintaining and increasing soil fertility by utilizing local, renew-able resources. The emphasis is on non-chemical disease prevention rather than treatment. Livestock plays an important role in providing manure and of course, marketable products. It is difficult to provide a general picture of organic farming in Europe. This is due to a diversity in farmers’ attitudes and a lack of uniformity in standards between countries. In a few countries like France and Denmark the rules governing organic standards are gov-ernment enforced but in most other places one or several private organizations provide the responsible authorities. In Denmark, the legislative rules of organic farming with relevance to parasite control are summarized in Table 1. These rules are in general accordance with proposed EU standards and the basic standards of the worldwide IFOAM (International Federation of Organic Agricultural Movements).
It is obvious that the rules are not very specific and there is ample room for different interpretations. A crucial aspect, of course, is how parasitic disease is diagnosed because many parasitic infections are ubiquitous and present a continuum of infection levels. Control is perhaps not a proper term as the ultimate goal is harmony between all living organisms on the farm. At first sight, this may be hard for us as traditional advisors and researchers to understand and accept but achieving a balance between pathogens and animals may in fact be a useful scientific goal. A more esoteric viewpoint supported by biodynamic farmers, is that worms aerate the contents of the gastrointestinal tract in a way similar to earthworms in the soil.
At the same time, conventional (as opposed to organic) livestock production has also changed, with the development of ever larger farm units. Growing consumer concern and increasing awareness among veterinarians of chemotherapeutic resistance have resulted in stricter control of use of veterinary medicines. In Denmark, new guidelines for veterinary treatments have been developed and anthelmintics will be put on prescription this year (1999).
This paper reviews available information and some theoretical considerations on para-sitic problems related to conversion from conventional to organic production and discusses options for control with focus on management, biological control and nutrition in conjunc-tion with or without chemicals. An extensive overview of alternative approaches to parasite control, including breeding of livestock for resistance, is not intended. The review focuses on northern temperate regions with the main emphasis on endoparasites.
2. Parasitological problems associated with conversion to organic livestock production: facts and fiction
As the conversion from conventional to organic farming affects management procedures and feeding in different ways depending on animal species, each livestock category will be considered separately. Hence, in this section little consideration will be made of the fact that integration of livestock species may result in a better balance within the agricultural system and is practised by many farmers. Most available data stem from farms of only a few years experience with organic practice. This may influence the results for a number of reasons: parasite infections have not built up, a proper rotation of fields has not been established and introduction of helminth species from the wild fauna has not yet occurred.
2.1. Ruminants
Dairy production is the system least affected by conversion and consequently little change is expected in the spectrum of parasites. More feed is homegrown on organic farms, the diet contains a higher proportion of roughage, mainly in the form of grass and silage, and the stocking rate is generally reduced. The increasing use in Denmark of deep litter barn systems for calves has resulted in observed problems with coccidia (Eimeria zuerni and E.
bovis). Coccidiosis due to E. alabamensis on pasture or in yards has also been observed. The
organic practice of turning out calves at 3-4 months of age may be associated with a higher risk of parasitic gastroenteritis (Shaw et al., 1998). Strategic approaches to helminth control in calves (e.g. intra-ruminal boli and repeated pour-on treatments in the early grazing season) widely adopted in conventional systems are not permitted. A survey of Danish organic farms revealed disease producing levels of gastrointestinal nematode infections affecting 7–32% of heifers on 5 farms out of 11, and D. viviparus infection was detected on three farms (Vaarst and Thamsborg, 1994). In this study, cattle had been moved to new pastures several times during the season and feed supplementation had been given. In all cases, management was determined by grass supply and not by parasitological considerations (Vaarst et al., 1996). However, the large area of clover and grass available within the rotation on organic farms offers good possibilities for implementing preventive or evasive strategies. A recent Swedish questionnaire study on parasite control in dairy herds demonstrated that grazing management procedures, primarily turning out onto clean pastures and grazing of aftermath, were widely adopted on organic farms in contrast to conventional farms (Svensson et al., 1998, unpublished data). Diarrhea in young cattle was reported in 14% and 6% of organic and conventional herds, respectively. Lungworm infections tend to be a recurrent problem of young stock and cows in both organic and conventional farms.
Beef production on grass using steers of dairy or mixed breed is common in many parts of the world but is presently of little importance in the Nordic countries. This may change in the years to come as the current practice of rearing of dairy bull calves indoors on slatted floors is subject to criticism on welfare grounds. On most organic farms, bull calves are sold due to lack of organically produced feed. Outdoor rearing of steers on permanent grassland without anthelmintics is an option although there obviously is a risk of parasitic disease (e.g. Thamsborg et al., 1998a). Observations from two experimental organic mixed sheep–cattle farms in New Zealand showed that acceptable parasite control can be achieved in sheep and to a lesser extent in cattle by alternate grazing, restricting anthelmintic treatments to disease outbreaks (Niezen et al., 1996). It was emphasized that a high level of planning and management was required to ensure adherence to the grazing systems.
2.2. Pigs
Outdoor pig production has a long tradition in some areas whereas intensive, indoor production systems have dominated in many West European countries for decades. In most recently developed outdoor systems, the sows are kept permanently on pasture where they farrow even in winter, while the large majority of growing pigs are moved indoors for the whole fattening period, e.g. to a deep litter system. A major difference between conventional, outdoor production and organic production is the high proportion of roughage, primarily silage, in the organic feed and, in most countries, a ban on routinely applied anthelmintics. Large differences exist in spectrum and intensity of endoparasitic infections between pig production systems. Thus, only Ascaris suum and Oesophagostomum spp. are common in traditional indoor systems in Denmark, while Trichuris suis and Strongyloides ransomi oc-cur only sporadically (Roepstorff et al., 1998). Other species seem to have been eradicated from Danish swine during the last 20 years due to almost exclusive indoor pig produc-tion: Metastrongylus spp. because of the need of an intermediate host (earthworms), and
Hyostrongylus rubidus perhaps because of the environmental requirements of the
devel-oping larvae, since infections are largely restricted to outdoor herds (Connan, 1977). The prevalence rates and infection intensities of the most common helminth species in the dif-ferent age groups of pigs are strongly influenced by their immunogenicity. Thus, A. suum and T. suis, which elicit a strong immune response, are most common in growing pigs and the infection levels decline in older pigs due to acquired immunity (see Nansen and Roep-storff, 1999). A recent study suggests that experimental A. suum infection of sows during gestation may induce tolerance in the neonatal piglets (Boes et al., 1999) and thus facilitate vertical transmission of the infection. This observation needs to be confirmed under natu-ral conditions. S. ransomipredominates in young pigs, viz. piglets and weaners, due to its transcolostral transmission from sows to piglets in combination with the fast development of acquired resistance (see Nansen and Roepstorff, 1999). In contrast, Oesophagostomum spp. and H. rubidus show the highest prevalence and intensity of infection in the older, breeding stock, which is presumably a reflection of a lower immunogenicity of these species. Pig coccidia include Isospora suis that predominates in young piglets (highly immunogenic) and Eimeria spp. that are most common in older pigs particularly in pigs having access to outdoor runs (Roepstorff et al., 1998). Consequences of the conversion to organic, largely
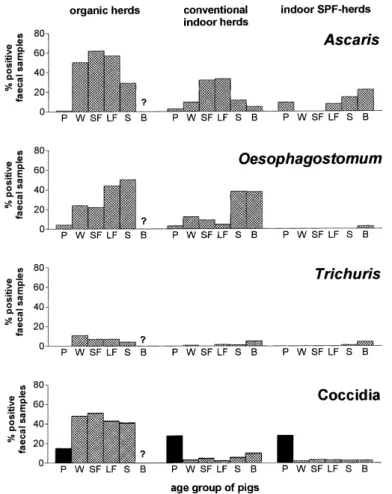
Fig. 1. Infection levels in pigs in different production systems. Organic farms represented pioneers in organic pigs production, and some had limited experience in animal husbandry and pasture rotation. Indoor intensive production is characterized by slatted floor, no strawbedding and strict sectioning of the production. P: piglets max. 5 weeks old; W: weaners; SF: small fatteners; LF: large fatteners; S: sows; B: boars. Coccidia: black columns predominantly Isospora; crosshatched columns predominantly Eimeria. Modified from Nansen and Roepstorff (1999) (data from Roepstorff et al., 1992, 1998).
outdoor production include generally higher infection levels and earlier acquisition of the infections (Fig. 1). Thus, approximately 50% of organic weaners were found to be excreting
A. suum eggs by 10 weeks of age, indicating acquisition of infection within the first few
weeks of life, while few or none of the conventionally reared indoor weaners of this age were coprologically positive. I. suis has been reported to be most prevalent in intensive indoor herds (Roepstorff and Nilsson, 1991). One of the organic herds studied by Roepstorff et al. (1992) started as an intensive indoor herd with a 54% prevalence of I. suis in the piglets, while no oocysts were found in this age group after the conversion to outdoor farrowing.
Helminth transmission rates under outdoor conditions depend on the pattern of devel-opment and survival of the free-living stages. While O. dentatum eggs may develop to infectivity within 1–2 weeks during a Danish summer, A. suum eggs need at least 4 weeks
(Roepstorff and Murrell, 1997; Mejer et al., 1998). Some eggs of T. suis may be infective within 8 weeks (Mejer et al., 1998) but the majority of the eggs do not become infective until the following summer (Burden and Hammet, 1979; Larsen and Roepstorff, 1999). None of the eggs/larvae develop within the winter period, as the lower threshold of development is somewhere between 10 and 17◦C for the three species (see Nansen and Roepstorff, 1999).
Ascaris and Trichuris, characterized by larval development within highly resistant eggs,
remain infective under outdoor temperate conditions for upto 6 and 11 years, respectively (Müller, 1952/1953; Burden et al., 1987). Consequently, a pasture that is clean in the au-tumn will remain non-infective until the spring irrespective of the level of contamination during the winter. However, accumulated eggs of A. suum and T. suis will start develop-ing when the temperature rises and the pasture may therefore become highly infective in the early summer (Larsen and Roepstorff, 1999). Although very resistant to environmental factors, A. suum and T. suis eggs may be subjected to a high mortality when eggs in faeces are exposed to desiccation and fluctuating temperatures during dry summer (Larsen and Roepstorff, 1999). Nevertheless, the transmission rate of A. suum is in general still high in outdoor systems using permanent pastures. Oesophagostomum eggs deposited on a pasture in the winter will die (Larsen, 1996) and although some infective larvae do survive outdoors during winter in temperate regions (Haupt, 1969), practical experience now shows that even highly infective pastures may be totally free of larvae by the following spring (Smith, 1979; Mejer et al., 1998).
What are the implications of these changes in infection patterns for the health and produc-tivity of pigs? Apart from isosporiosis, endoparasitic infections of indoor pigs are generally subclinical by nature (see Hale and Stewart, 1987). A few case reports of severe clinical strongyloidosis, trichuriosis and eimeriosis in pigs turned out on heavily contaminated out-door enclosures do exist (Grini, 1937; Alfredsen and Helle, 1980; Jensen and Svensmark, 1996). Experimental studies have indicated that subclinical Oesophagostomum spp. infec-tions in penned sows may cause reduced litter size and growth rate of the piglets (Pattison et al., 1979; Ferber and Thomas, 1980), while similar studies on other helminths have not been carried out. A factor of unknown importance is the introduction of new species in outdoor systems, e.g. H. rubidus. Helminth infection as a contributory cause in other diseases has been demonstrated by Mansfield and Urban (1996) associating severe bacterial colitis with
T. suis induced immunosuppression. In conclusion, the significance of helminth infections
with regard to health and productivity in pigs under outdoor farming conditions remains to be documented.
The potential risk of introduction of a zoonotic helminth like Trichinella spp. in outdoor pig production will depend on the prevalence of the infection in the wild fauna of the area, pasture management and other preventive measures on the farm e.g. sanitary disposal of dead animals and rat and cat control (Pozio, 1998). The increase of Trichinella infections in pigs in the Baltic countries in the last decades indicates that this risk is real (Kapel, 1997). However, changes in prevalence associated with organic farming practices remain to be documented.
2.3. Poultry
The conversion to organic egg production includes changes in housing and feeding. All laying hens must have access to outdoor yards or paddocks (2.5–4 m2/hen) thereby creating
a natural environment for transmission of parasite infections. Accordingly, more species of helminths and heavier worm burdens have been found in hens reared organically or free range compared to caged and deep litter hens. In Denmark, prevalences of Ascaridia
galli were 64, 42 and 5% in organic/free ranging, deep litter and battery cage systems,
respectively. Corresponding figures for Heterakis gallinarum were 73, 19 and 0% and
Capillaria obsignata 54, 52 and 0% (Permin et al., 1999). C. anatis and C. caudinflata
were present only in organic/free ranging flocks. In this study, each production system was represented by four farms only, but the results suggest that the risk of a hen becoming infected is much higher on organic farms than in caged systems. However, the significance of these infections in terms of disease and production losses on organic farms has not been assessed. H. gallinarum may play an important role as it transmits Histomonas meleagridis, which has recently been found in Danish organic farms. Similarly, it has been shown that
A. galli eggs may act as mechanical vectors of salmonella (Chadfield et al., 1997). Heavy Ascaridia infections may thus affect the transmission of salmonella within the flock. The
ban on coccidiostats in the feed on organic farms (cf. Table 1) may also have serious implications for the health of young replacement stock and broilers but information is not presently available.
3. Integrated and non-chemotherapeutic control
The term integrated pest control implies a rational use of a combination of biologi-cal, biotechnological and chemical control measures with farming practices or breeding strategies in order to reduce the use of chemical control agents to an absolute minimum. A classical example of this approach is the combination of grazing management and an-thelmintic treatment as introduced in the Weybridge dose and move system for cattle in the sixties (Michel, 1969). The relevance of an integrated approach has grown for several reasons. A single anthelmintic treatment of an animal in an infected environment proved to have a very transitory effect as it becomes reinfected shortly after treatment (disregard-ing the macrocyclic lactones). The spar(disregard-ing use of parasiticides has been advocated due to increasing problems with chemical resistance. A combination of two or more less effec-tive methods may substantially reduce infection levels and give appropriate control. Lastly, under some conditions, a control programme of very high efficacy, e.g. use of sustained release devices, may be unwanted as it may compromise the development of immunity. Protected animals may thereby remain susceptible to disease if exposed to infection at an older age or after the end of treatment. It may be added that any efficient control system may lead to ‘underexposure’. In organic farming systems, preventive use of parasiticides is not an option. Even so, an integrated approach combining different methods is likely to achieve the best control. Documentation of these options is much more extensive in ru-minants than in monogastric animals and the following paragraphs will consider possible strategies of integrated or non-chemotherapeutic control in both conventional and organic farming systems.
3.1. Management of pastures and yards
Grazing management procedures to control helminth infection are often grouped as pre-ventive, evasive or diluting (Barger, 1997). Preventive procedures include the introduction of uninfected animals to a parasite free or clean area and the build up of infections is thus prevented. Clean areas may be new leys or can be provided on permanent pasture by alter-nation with other species not sharing the same spectrum of parasites, by spelling of pastures or use of aftermath after harvesting hay or silage crop. The classical preventive situation is turning out previously unexposed stock e.g. young heifers or older treated stock onto clean pastures but an all-in-all-out production system where new areas are provided for each batch of animals, e.g. free ranging broilers, may likewise be regarded as preventive. Evasive strategies aim at avoiding disease producing infections of a contaminated area by moving stock to a clean area. This can be carried out one or several times in the produc-tion life of an animal e.g. during a grazing season and can be combined with anthelmintic treatment in conventional systems. Dilution is a means of non-chemotherapeutic control and can be achieved by mixing young susceptible stock with another species, with older resistant stock or simply by reducing the animal density or stocking rate i.e. the number of animals per unit area. The efficacy of mixed or alternate grazing will depend on the ratio between susceptible and inert stock e.g. the sheep–cattle ratio on the farm. In general, a lower stocking rate is maintained on organic farms as a proportion of the grazing area has to be used for nitrogen-fixing plants, particularly clover grass, when commercial fertilizer is not used.
3.1.1. Preventive and evasive procedures
Preventive and evasive procedures are likely to play an important role in future parasite control, particularly in monogastric animals where other options are lacking or are not fully investigated. In outdoor poultry production systems, anthelmintic treatments are often not appropriate due to egg and slaughter withdrawal periods. Approaches are therefore generally preventive. Provision of clean pasture and cleaning of any permanent facilities for each batch or production year are necessities but may not exclude the build up of infections. The time needed for resting pastures between batches to prevent transmission is debatable but one year has been laid down in the new Danish organic standards.
When establishing a new outdoor swine herd, stock may be purchased from intensive indoor herds to limit the risk of introducing parasites, particularly Oesophagostomum and
Trichuris (and ectoparasites). It is a common strategy to move the farrowing huts between
farrowings and this may reduce the prevalence of I. suis infections in the piglets substantially (Roepstorff et al., 1992). In conventional outdoor sow herds, weaners are treated with anthelmintics when they are introduced into the fattening unit. Since free-living stages of Oesophagostomum spp. do not survive outdoors very well through temperate winters, pastures free (or relatively free) of this helminth are easily provided. In contrast, pasture rotation as a means of control of A. suum and T. suis in outdoor pigs may seem futile due to the extreme longevity of the eggs. However, Larsen and Roepstorff (1999) found that 80–99% of the eggs disappeared within a few months of deposition on a pasture, i.e. before the large majority of eggs reached infectivity. It is thus likely that a systematic pasture
rotation may have a profound effect on the transmission rate of pig helminths in general but controlled studies are still lacking. The need for anthelmintic treatment in association with pasture management on conventional farms or the number and timing of moves on organic farms are not known. Regarding Oesophagostomum, efficient anthelmintic treatment (s) of all pigs in late winter followed by grazing of clean pastures in early spring, may possibly eradicate the helminth from a herd.
In some countries, it is common to apply a nose-ring to sows in outdoor herds, including organic herds, thereby reducing their rooting behaviour and thus securing the grass sward as a fodder supply for a longer period. As the survival of both Oesophagostomum larvae (Larsen, 1996) and Ascaris and Trichuris eggs (Larsen and Roepstorff, 1999) is better in soil compared to a short sward, nose-rings could be a contributory factor to the very low infection levels found in several Danish outdoor sow herds not using anthelmintics (Roepstorff et al., 1992). However, a recent trial failed to show any significant effect of nose-rings on helminth transmission (Mejer et al., 1998).
In cattle, the concept of dosing first year grazing heifers in mid-July and moving them to clean pasture has proved to be efficacious under northern temperate conditions (Eysker et al., 1998a). This principle is also advocated under different epidemiological conditions in Australia (e.g. Axelsen et al., 1986). Apart from possible implications for the development of anthelmintic resistance, the major risks associated with this procedure in northern temperate areas include early season parasitic gastroenteritis and the build up of infections in late season. The latter risk is particularly high in grazing seasons with a dry summer when the major contamination of the pasture is postponed until autumn rainfall occurs. Early season gastroenteritis is often seen in the subsequent season if the winter is cold (Nansen et al., 1989; Eysker et al., 1998a). Studies have shown an almost similar beneficial effect when cattle have been moved to clean pasture without any anthelmintic treatment (Foldager et al., 1981; Nansen et al., 1988), but repeated moves may be necessary under practical conditions e.g. in an extended grazing season. Eysker et al. (1998b) achieved effective control of nematode infections of first season heifers by three moves to clean pasture but emphasized that the last move should not be more than one month before housing.
A dose and move strategy has also been devised for small ruminants (Barger, 1997). This is recommended for ewes with lambs at the time of weaning, e.g. in the UK (Coles and Roush, 1992). Weaners often have to be moved to clean pasture a second time with or without treatment. A non-chemotherapeutic approach involving repeated moves has shown good efficacy under tropical conditions grazing goats continuously on a rotational basis with 7 paddocks/areas and a move every 3.5 days (Barger et al., 1994). The survival of infective larvae (primarily Haemonchus) on the pasture was estimated to be only 3–7 weeks under these conditions. Under temperate conditions with much longer survival times for infective larvae, particularly Nematodirus, this approach is not likely to prove successful. In ewes with lambs, a single move by 1 July without treatment limited Ostertagia/Trichostrongylus infections in lambs post-weaning but had no effect on Nematodirus spp. under Danish conditions (M. Boa and S. M. Thamsborg, unpublished data).
Alternation of sheep and cattle on an annual basis or more often is another preventive strategy. It is evident that this strategy with no, or limited, use of anthelmintics results in effective nematode control and good weight gains in the sheep (Donald et al., 1987; Niezen et al., 1996). However, the productivity of the cattle is often not satisfactory (Niezen et
al., 1996) and in some cases higher worm burdens in calves may result (Bairden et al., 1995). In the latter study lasting 4 years, the rotation was annual and it was suggested that the infective larvae of Cooperia oncophora and Ostertagia ostertagi either survived for prolonged periods on pasture or were propagated through the sheep. A three-year rotation with sheep followed by cattle and an arable crop may achieve better control. It seems that the adaptation of previously host specific helminths to other hosts is a substantial threat to the sustainability of alternate (and mixed) systems as argued by Barger (1997).
3.1.2. Diluting procedures
Mixing young and older, parasitologically inert stock may be relevant for cattle and perhaps sheep (weaners and dry ewes). Nansen et al. (1990) showed that mixing equal numbers of first and second season dairy heifers prevented parasitic gastroenteritis in the former as compared to first season heifers grazing alone. Although fecal egg counts were similar, first season heifers in the mixed group performed better. A potential risk associated with this strategy is introduction of nematode infections, particularly D. viviparus,with second season grazers in spring if animals are not effectively dewormed during winter. A survey of 11 organic dairy farms revealed positive egg counts in 41% (max. 240 epg) of second season grazers at turnout (M. Vaarst and S. M. Thamsborg, unpublished data). Grazing studies with ewes + lambs and cows + calves over a three-year period revealed reduced worm counts and better weight gains in lambs in mixed grazing systems (Jordan et al., 1988) but calves had lowered productivity and worm burdens were not reduced. This is very similar to observations in alternate grazing systems (see above).
A recent study of mixed (and alternate) grazing with nose-ringed sows and heifers, showed promising results in controlling Ostertagia infections in the cattle whereas little effect on the nematode infections of sows were noted (A. Roepstorff and J. Monrad, unpublished data). The lush grass surrounding the fecal pats typical of cattle grazing was absent due to the sows grazing and spreading and eating of cattle feces. In warm moist environments, however, it cannot be excluded that this spreading of faeces may lead to higher herbage infectivity.
Few studies have investigated the effect of stocking rate on helminth infections of mono-gastric animals in out-door areas. A study in laying hens using stocking rates of 3, 5 and 10 hens/m2 failed to show any effect on A. galli infections (Permin et al., 1998). In the first year of a two-year study using stocking rates of 17, 42 and 100 weaner pigs per ha, significantly higher fecal egg counts and O. dentatum worm burdens were recorded at the high stocking rate compared to the medium and low stocking rates, while stocking rate did not correlate with A. suum and T. suis infection levels (Mejer et al., 1998).
In contrast to pigs that are living primarily of supplementary fodder, the situation is fundamentally different in grazing ruminants where a change in stocking rate will under most circumstances lead to a change in forage/feed availability, e.g. a higher stocking rate will result in less feed available per animal. This complicates the situation as it is well known that the nutritional level affects establishment and fate of parasite infections. Several studies have indicated a positive association between stocking rate and nematode fecal egg counts or worm burdens in dairy heifers (Hansen et al., 1981; Nansen et al., 1988), in more extensive beef production (Ciordia et al., 1971; Donald et al., 1979; Thamsborg et al., 1998a)
and in sheep (Downey, 1969; Brown et al., 1985; Thamsborg et al., 1996), whereas others have failed to show an association (e.g. Hansen et al., 1989). The relationship between stocking rate and parasitic infections seems to depend on the age group of the animals, climate, initial infection levels and nematode species involved. Thus, the above cited sheep studies all report on lambs postweaning infected with Trichostrongylus or Nematodirus spp. leading to higher levels of nematode associated morbidity with increasing stocking rate after two or more years. In the most recent study (Thamsborg et al., 1998b), this effect was seen even though pasture productivity of the high stocking rate pastures was enhanced by higher levels of fertilizer and the animals were not nutritionally compromised at any level of stocking rate. This result contrast reviews of Michel (1969) and Barger (1978) hypothesizing that if stocking rate was increased, and plant production was increased proportionally by means of fertilizer according to sound farming practice, nematode infections would not cause increased pathology or decreased weight gains. On the contrary, it now appears that nematode infections may cause severe problems in lamb production under conditions of heavy fertilizer application and intensive grazing. It may, however, be argued that adjustment of stocking rate is not a control procedure per se but may rather be viewed upon as a risk factor (Barger, 1997; Shaw et al., 1998).
3.1.3. Composition of feed and pastures and botanical dewormers
Studies on the interactions between nutrition and ruminant nematode infections are nu-merous and will not be reviewed in extenso in the present paper. However, a few recent observations will be considered. Certain leguminous plants with a high content of condensed tannins seem to affect nematode infections or improve performance of parasitised lambs. Worm burdens in lambs have been reduced by up to 50% depending on species by feeding sulla (Hedysarum coronarium) or great trefoil (Lotus pedunculatus) (Niezen et al., 1995, 1998). Concentrations of condensed tannins of less than 10% of dietary DM are believed to protect plant protein against ruminal degradation, thus increasing the protein availability in the small intestine and improving the animal’s protein supply (Waghorn et al., 1997). However, it has been difficult to relate anti-parasitic effects to the actual amounts of con-densed tannins. The possible mode of action is largely unknown and the results after adding polyethylene glycol, a compound binding and blocking the effects of condensed tannins, have not been conclusive (e.g. Niezen et al., 1998). Enhanced immunity is hard to envisage in view of the short duration of reported studies and a more direct anthelmintic effect is perhaps more likely. A further complication is the fact that condensed tannins are a poorly defined group of compounds making standardized determinations in plant material diffi-cult. Furthermore, the contents of condensed tannins are variable depending on soil type, rainfall and cultivars. However, the concept of using pasture species with a possible effect on nematode infections in grazing management looks promising and the possibilities seem far from exhausted. Plants that can be established locally and used as part of the normal feeding regime are most likely to be acceptable to farmers, particularly organic farmers. The plants can possibly be used on a temporal basis (short term stay in a ‘deworming’ paddock) or they can be mixed with grass and clover in the whole grazing area. Interestingly enough mixtures of herbs and grasses are common practice in biodynamic farming. In line with this is research into the usage of locally available botanical dewormers for mainly therapeutic
treatment (cf. Danø and Bøgh, 1999). It is unlikely that remedies used traditionally have serious side effects but one should still be cautious substituting existing well defined chem-ical anthelmintics with lesser known herbs for therapy and secondly, the issue of residues in the products should be considered. The use of homoepathy lacks scientific documentation at present.
Several components in the diet may affect nematode infections but relatively few studies have been carried out in monogastric animals. In pigs, high levels of insoluble dietary fibres have resulted in higher establishment rates and better fecundity of O. dentatum compared to diets of similar protein and energy levels but rich in digestible carbohydrates and proteins (Petkevi˘cius et al., 1999). A. suum infections were not affected in a similar way. These findings may have important implications for the epidemiology of Oesophagostomum spp. in sows under organic farming conditions. In such systems, they are usually permanently exposed to infection, and roughage, primarily fresh grass or whole grain silage is fed ad libitum. Interestingly, feed structure also affects bacterial infections as commercial pelleted feed increases the prevalence of salmonella compared to homegrown feed.
3.2. Biological control
Biological control may be defined as the use of one living microorganism introduced into the environment to obtain control of a target microorganism in casu the target parasite, and thereby reducing the population growth of the latter below a threshold where it causes clinical problems and/or economic losses. Biological control of parasitic nematodes in livestock therefore aims at establishing a situation where the grazing animals are exposed to a low level of infective larvae, but at a level that will secure the development of naturally acquired immunity in the same animals. Among the naturally occurring enemies of free-living nematodes, including pre-parasitic larval stages on pasture, only predacious micro-fungi have been extensively tested so far. These nematophagous micro-fungi are able to reduce populations of pre-parasitic nematodes significantly, are relatively easily cultured and can be released in the environment of the target organisms in a controlled fashion.
3.2.1. Activity against nematode infections of ruminants and monogastric animals
Several field trials with set-stocked animals, performed primarily in the northern hemi-sphere, have clearly demonstrated that the fungus Duddingtonia flagrans is very efficient in controlling most of the economically important gastro-intestinal parasites of grazing live-stock by reducing pasture infectivity (Larsen, 1999). In ruminants, the experimental use of
D. flagrans in dairy cattle heifers is particularly well documented whereas only few reports
of use in small ruminants are available. The approach has been primarily strategic by group feeding with the fungal spores daily from 2–3 weeks after turnout for 2–3 months i.e. un-til after the expected mid summer rise in pasture infectivity of gastrointestinal nematodes (Larsen et al., 1995; Nansen et al., 1995; Grønvold et al., 1993). This work has more recently been confirmed in other places e.g. Lithuania (Sarkunas et al., unpublished data). Infections with the cattle lungworm, D. viviparus, are also of major concern in both organic and con-ventional herds and D. flagrans has recently been shown to reduce significantly transmission from artificial dung pats in laboratory studies (Henriksen et al., 1997) and from dung to
herbage in plot studies (A.S. Fernandez et al., unpublished data). However, it has yet to be shown whether the doses of fungal spores used for controlling gastrointestinal nematodes in field trials are also able to limit D. viviparus infections. Activity against other nematodes with a different transmission biology also requires investigation. The recent observation that D. flagrans reduce Nematodirus battusinfections of lambs is of significance as these eggs have special requirements for hatching and the larvae are active at a different time of year (Githigia et al., 1997). In pigs, a single field trial has shown significant reductions in acquired Oesophagostomum spp. and H. rubidus infections by adding D. flagrans to the feed (Nansen et al., 1996). The effect of D. flagrans in faecal cultures from pigs does not seem to be affected by different levels of insoluble dietary fibre in the feed (Petkevi˘cius et al., 1998).
The activity of nematophagous fungi investigated has so far been restricted to nematode species with larval stages in feces. Other biological control agents are needed for nematodes with egg dwelling infective stages. These eggs often last for a long time (years) in the environment, such as A. galli of poultry and A. suum and T. suis of pigs. Egg parasitic fungi are presently being investigated, but no convincing results have yet been published. If successful, direct treatment of deep litter could be an attractive option for these infections and the addition of such agents to the control repertoire would widen the potential for biological control, particularly in pigs and poultry. Nematode destroying fungi, either as a single species or a mixture of species and types (trapping and egg parasitic), could perhaps in the future be used also for hygienic treatment of human waste where this is used as fertilizer.
3.2.2. Nematophagous fungi in integrated control
Nematophagous fungi reduce the number of infective larvae developing in feces but have no effect on larvae already passed to the vegetation or on worm burdens in the animals. This was clearly demonstrated in a study by Githigia et al. (1997) in which weaner lambs suffered from parasitic gastroenteritis due to heavy overwintering infections after one month of fungi feeding in early season. Therefore, to maximize the effect of nematophagous fungi, or any other biological control agent, it is important first to secure a low level of pasture infectivity by grazing management strategies or by strategically applied anthelmintic treatments. It is anticipated that after animals have been dosed for a couple of seasons, the larval population on pasture will be diminished and the need for anthelmintic intervention reduced, but these long term effects still remain to be tested. Also, the use of nematophagous fungi as an element of integrated control strategies needs further consideration e.g. combining a move to clean pasture with a shorter period of fungal dosing.
At present, impediments to the adoption of nematophagous fungi in practical control schemes include a lack of suitable application systems, assessment of long term environ-mental effects and finally, acceptance of the principle by farmers. Experience from field trials indicates that mixing the fungal chlamydospores into a feed supplement is a realistic option for long term application. Also incorporation into various types of feed blocks or mineral licks may become a feasible option. Both types of dosing system could be applied on organic as well as conventional farms with intensive production systems, where daily feeding can secure a sufficient daily fungal dose. In more extensive systems, feed blocks or licks would be preferred but there may be variability due to differences in voluntary intake.
If technically feasible, incorporation into a ruminal bolus would significantly increase po-tential usage on conventional farms but this possibility remains to be investigated. Spreading a large amounts of fungal spores in a restricted environment, e.g. in a deep litter pen, may prove successful in the control of Strongyloides spp. of housed cattle (Chandrawathani et al., 1998).
4. Conclusions: parasite control in a ‘post-anthelmintic era’
It is clear that the rapidly increasing number of organic farms will give rise to a resurgence in endoparasitic problems, particularly in poultry and pig production. In ruminants, the well-known problems of trichostrongyle parasites have to be solved by non-medical means on these farms but for obvious reasons, alternative approaches are also needed in many conven-tional production systems. The impact of organic farming on parasitic infections is not only associated with the withdrawal of preventive drug therapy. Many management procedures are fundamentally different and may influence biological relationships in unexpected ways e.g. the influence of diet on Oesophagostomum spp. infections in pigs. This is an interesting challenge for today’s veterinary parasitologist.
Pertinent information is urgently needed by extension workers advising farmers who are converting to organic production. Studies on parasitic problems need to be performed on commercial organic farms for several reasons. Farmers’ attitudes and management decisions may differ from those in traditional enterprises. Grazing management, which is likely to play an important role in control, does in contrast to a chemotherapeutic approach depend on the specific crop rotation and composition of stock on the farm. The required extra planning and management procedures make acceptability to farmers imperative for widespread adoption. Future research should include the identification of ‘new’ parasitic problems in groups of livestock such as sow, piglets, fatteners, egg laying hens and broilers reared outdoor or in deep litter systems. Assessing risk factors and impact on health and production must be given high priority in organic farming. Acceptance of a certain degree of production loss without compromising welfare may be an option, in contrast to more industrialized production. A single, but important issue, is the risk assessment of parasitic zoonoses in large outdoor rearing pig units.
On cattle enterprises, turning young calves out onto lightly infected pastures in spring with subsequent repeated moves may prove to be effective. There are some indications that this may also control D.viviparus infections (Eysker et al., 1995). Permanent paddocks or grassland should never be used for first season grazing stock at turnout, primarily due to the risk of coccidiosis. In pig and poultry production, introduction of new stock onto clean pastures or after having removed the bedding in deep litter systems is regarded as essential in parasite control. However, the need for further moves and rotational schemes is largely unknown. The use of different species in mixed or alternate grazing systems rotated with crops, although limited by availability of stock, needs further examination. Regarding biological control, it is essential that technology which allows practical and economical administration of nematophagous fungi to livestock in the farm setting be developed. Re-search effort should be focussed on obvious delivery mechanisms such as the slow release bolus or feed blocks.
In conclusion, the prospects for the control of many nematode infections in a ‘post-anthelmintic era’ are good, as judged from the experimental evidence. However, develop-ment and impledevelop-mentation of control strategies acceptable to farmers in different production systems, including organic, are still needed. The oldest and simplest way of dealing with parasitic infections is to use drugs when clinical disease appears. It is well known that this approach will be accompanied by heavy production losses in the herds and that it is highly questionable from a welfare point of view. Securing sufficient exposure to induce immunity in young stock through an integrated approach based primarily on grazing man-agement, perhaps supported by herd monitoring, seems more likely to be successful. In the longer term, grazing management can be supplemented by biological control or the use of specialized crops which reduce infection levels.
References
Alfredsen, S.A., Helle, O., 1980. Coccidiose hos gris. Norsk. Vet. Tidsskr. 92, 36–38.
Anon. 1998. Ecological production. Directive No. 210, 6. April 1998, of the Danish government.
Axelsen, A., Waller, P.J., Donald, A.D., Dobson, R.J., Nadin, J.B., 1986. Grazing management and nematode parasite control in cattle in the temperate climatic zone of Australia. Aust. J. Exp. Agric. 26, 267–273. Bairden, K., Armour, J., Duncan, J.L., 1995. A 4-year study on the effectiveness of alternate grazing of cattle and
sheep in the control of bovine parasitic gastro-enteritis. Vet. Parasitol. 60, 119–132.
Barger, I., 1978. Grazing management and control of parasites in sheep. In: Donald, A.D., Southcott, W.H., Dineen, J.K. (Eds.), The Epidemiology and Control of Gastrointestinal Parasites of Sheep in Australia, CSIRO, Melbourne, pp. 53–63.
Barger, I.A., 1997. Control by management. Vet. Parasitol. 72, 493–506.
Barger, I.A., Siale, K., Banks, D.J.D., Le Jambre, L.F., 1994. Rotational grazing for control of gastrointestinal nematodes of goats in a wet tropical environment. Vet. Parasitol. 53, 109–116.
Brown, T.H., Ford, G.E., Miller, D.W., Beveridge, I., 1985. Effect of anthelmintic dosing and stocking rate on the productivity of weaner sheep in a Mediterranean climate environment. Austr. J. Agric. Res. 36, 845–855. Boes, J., Medley, G.F., Coates, S., Varady, M., Eriksen, L., Roepstorff, A., Nansen, P., 1999. The effect of maternal
exposure on experimental Ascaris suum infections in suckling pigs, Parasitology, submitted for publication. Burden, D.J., Hammet, N.C., 1979. The development and survival of Trichuris suis ova on pasture plots in the
south of England. Res. Vet. Sci. 26, 66–70.
Burden, D.J., Hammet, N.C., Brookes, P.A., 1987. Field observations on the longevity of Trichuris suis ova. Vet. Rec. 121, 43.
Chandrawathani, P., Omar, J., Waller, P.J., 1998. The control of the free-living stages of Strongyloides papillosus by the nematophagous fungus, Arthrobotrys spp. Vet. Parasitol. 76, 321–325.
Chadfield, M, Permin, A, Bisgaard, M, 1997. Investigation of the parasitic nematode Ascaridia galli as a potential vector for Salmonella dissemination in poultry. Salmonella and salmonellosis, Proc. Int. Symp., Ploufragan, May 20–22, 1997, France, pp 375–376.
Ciordia, H., Neville, W.E., Baird, D.M., McCampbell, H.C., 1971. Internal parasitism of beef cattle on winter pastures: levels of parasitism as affected by stocking rates. Am. J. Vet. Res. 32, 1353–1358.
Coles, C.G., Roush, R.T., 1992. Slowing the spread of anthelmintic resistant nematodes of sheep and goats in the UK. Vet. Rec. 130, 505–510.
Connan, R.M., 1977. The prevalence of Hyostrongylus rubidus. Vet. Rec. 100, 242–243.
Danø, R., Bøgh, H.B., 1999. Usage of herbal medicine against helminths in livestock. An old tradition gets its renaissance, World Animal Review, in press.
Donald, A.D., Axelsen, A., Morley, F.H.W., Waller, P.J., Donnelly, J.R., 1979. Growth of cattle on phalaris and lucerne pastures II. Helminth parasite populations and effects of anthelmintic treatment. Vet. Parasitol. 5, 205–222.
Donald, A.D., Morley, F.H.W., Axelsen, A., Donnelly, J.R., Waller, P.J., 1987. Integration of grazing management and anthelmintic treatment for the control of nematode infection in young sheep. In: Wheeler, J.L., Pearson, C.J., Robards, G.E. (Eds.), Temperate Pastures: Their production, Use and Management, CSIRO, Melbourne, pp. 567–569.
Downey, N.E., 1969. Grazing management in relation to Trichostrongylid infestation in lambs. Level of infestation associated with increased stocking rate and its effects on the host. Irish J. Agric. Res. 8, 375–395.
Eysker, M., Boersema, J.H., Cornelissen, J.B.W.J., Koyman, F.N.J., 1995. Efficacy of Michel’s dose and move system against Dictyocaulus viviparus infections in cattle using moxidectin as an anthelmintic. Vet. Parasitol. 58, 49–60.
Eysker, M., van der Aar, W.M., Boersema, J.H., Dop, P.Y., Koyman, F.N.J., 1998a. The efficacy of Michel’s dose and move system on gastrointestinal nematode infections in dairy calves. Vet. Parasitol. 75, 99–114. Eysker, M., van der Aar, W.M., Boersema, J.H., Githiori, J.B., Koyman, F.N.J., 1998b. The effect of repeated
moves to clean pasture on the build up of gastrointestinal nematode infections in calves. Vet. Parasitol. 76, 81–94.
Ferber, M.E., Thomas, R.J., 1980. The effect of Oesophagostomum quadrispinulatum infection on reproductive performance in the sow, Proc. IPVS, Copenhagen, p. 264.
Foldager, J., Sejrsen, K., Brolund Larsen, J., Nansen, P., Jørgensen, R.J., Hansen J.W., 1981. Control of infection with gastrointestinal helminths in calves and heifers on pasture, 514. Beretning fra Statens Husdyrbrugsforsøg, Frederiksberg, 325 pp.
Githigia, S.M., Thamsborg, S.M., Larsen, M., Kyvsgaard, N.C., Nansen, P., 1997. The preventive effect of the fungus Duddingtonia flagrans on trichostrongyle infections of lambs on pasture. Int. J. Parasitol. 27, 931–939. Grini, O., 1937. Strongyloides papillosus (Suis, Lonhus) hos griser. Norsk Vet. Tidsskr. 49, 1–13.
Grønvold, J., Wolstrup, J., Nansen, P., Henriksen, S.A., Larsen, M., Bresciani, J., 1993. Biological control of nematodes parasites in cattle with nematode-trapping fungi—a survey of Danish studies. Vet. Parasitol. 48, 311–325.
Hale, O.M., Stewart, T.B., 1987. Feed and maintenance costs of internal parasites in growing-finishing swine. Agri-Practice 8, 33–35.
Hansen, J.W., Nansen, P., Foldager, J., 1981. The importance of stocking rate on the uptake of gastrointestinal nematodes by grazing calves. In: Nansen, P., Jørgensen. R.J., Soulsby, E.J.L. (Eds.), The Epidemiology and Control of Nematodiasis in Cattle. Proc. of the CEC Workshop, Copenhagen. Martinus Nijhoff, The Hague, pp. 471–494.
Hansen, J.W., Zajac, A.M., Eversole, D.E., Gerken Jr., H.J., 1989. The effect of stocking rate and parasite control on the performance of replacement beef heifers on pasture. Vet. Parasitol. 34, 103–115.
Haupt, W., 1969. Ein Beitrag zur Überwinterungsfähigkeit der dritten invasionsfähigen Oesophagostomum-larven des Schweines auf der Weide. Archiv für Experimentelle Veterinärmedizin 23, 1211–1215.
Henriksen, S.A., Larsen, M., Grønvold, J., Nansen, P., Wolstrup, J., 1997. Nematode-trapping fungi in biological control of Dictyocaulus viviparus. Acta Vet. Scand. 38, 175–179.
Jensen, T.K., Svensmark, B., 1996. Trichuriasis hos udendørs slagtesvin. Veterinær Inform. 2, 3–7.
Jordan, H.E., Philips, W.A., Morrison, R.D., Doyle, J.J., McKenzie, K., 1988. A 3-year study of continuous mixed grazing of cattle and sheep: parasitism of offspring. Int. J. Parasitol. 18, 779–784.
Kapel, C.M., 1997. Trichinella in arctic, subarctic and temperate regions: Greenland, the Scandinavian countries and the Baltic states. Southeast Asian J. Trop. Med. Health 28 (1), 14–19.
Larsen, M.N., 1996. Årstidsvariation i overlevelse, udvikling og spredning for 4 porcine parasitter på friland, M.Sc. Thesis, University of Copenhagen.
Larsen, M.N., Roesptorff, A., 1999. Seasonal variation in development and survival of Ascaris suum and Trichuris
suis eggs on pastures, Parasitol., in press.
Larsen, M., 1999. Biological control of helminths. Int. J. Parasitol. 29, 139–146.
Larsen, M., Nansen, P., Wolstrup, J., Grønvold, J., Henriksen, S.A., Zorn, A., 1995. Biological control of trichostrongylosis in grazing calves by means of the fungus Duddingtonia flagrans. Vet. Parasitol. 60, 321–330. Mansfield, L.S., Urban, J.F., 1996. The pathogenesis of necrotic proliferative colitis in swine is linked to whipworm
induced suppression of mucosal immunity to resident bacteria. Vet. Immunol. Immunopathol. 50, 1–17. Mejer, H., Thomsen, L.E., Wendt, S., 1998. Transmission af helminther hos grise på friland. Betydning af næsering,
Michel, J.F., 1969. The epidemiology and control of some nematode infections in grazing animals. Adv. Parasitol. 7, 211–283.
Müller, G., 1952/1953. Untersuchungen über die Lebensdauer von Ascarideeiern in Gartenerde, Zentralblatt für Bakteriologie Parasitenkunden Infektionskrakheiten und Hygiene 159, 377-379.
Nansen, P., Roepstorff, A., 1999. Parasitic helminths of the pig: Factors influencing transmission and infection levels, Int. J. Parasitol., in press
Nansen, P., Foldager, J., Hansen, J.W., Henriksen, S.A., Jørgensen, R.J., 1988. Grazing pressure and acquisition of O. ostertagi in calves. Vet. Parasitol. 27, 325–335.
Nansen, P., Steffan, P., Monrad, J., Grønvold, J., Henriksen, S.A., 1990. Effects of separate and mixed grazing on trichostrongylosis in first- and second-season grazing calves. Vet. Parasitol. 36, 265–276.
Nansen, P.J., Grønvold, J., Jørgensen, R.J., Henriksen, S.A., Foldager, J., Sejrsen, K., 1989. Outbreaks of early-season trichostrongylosis in calves in Denmark. Vet. Parasitol. 32, 199–211.
Nansen, P., Larsen, M., Grønvold, J., Wolstrup, J., Zorn, A., Henriksen, S.A., 1995. Prevention of clinical trichostrongylosis in calves by strategic feeding with the predacious fungus Duddingtonia flagrans. Parasitol. Res. 81, 371–374.
Nansen, P., Larsen, M., Roepstorff, A., Grønvold, J., Wolstrup, J., Henriksen, S.A., 1996. Control of
Oesophagostomum dentatum and Hyostrongylus rubidus in outdoor-reared pigs through daily feeding with
the microfungus Duddingtonia flagrans. Parasitol. Res. 82, 580–584.
Niezen, J.H., Waghorn, G.C., Charleston, W.A.G., 1998. Establishment and fecundity of Ostertagia circumcincta and Trichostrongylus colubriformis in lambs fed lotus (Lotus pedunculatus) or perennial ryegrass (Lolium
perenne). Vet. Parasitol. 78, 13–21.
Niezen, J.H., Waghorn, T.S., Charleston, W.A.G., Waghorn, G.C., 1995. Growth and gastrointestinal nematode parasitism in lambs grazing either lucerne (Medicago sativa) or sulla (Hedysarum coronarium) which contains condensed tannins. J. Agric. Sci. Camb. 125, 281–289.
Niezen, J.H., Charleston, W.A.G., Hodgson, J., Mackay, A.D., Leathwick, D.M., 1996. Controlling internal parasites in grazing ruminants without recourse to anthelmintics: approaches, experiences, experiences and prospects. Int. J. Parasitol. 26, 983–992.
Pattison, H.D., Smith, W.C., Thomas, R.J., 1979. The effect of sub-clinical nematode parasitism on reproductive performance in the sow. Anim. Prod. 29, 321–326.
Permin, A., Nansen, P., Bisgaard, M., Frandsen, F., Pearman, M., 1998. Studies on Ascaridia galli in chickens kept at different stocking rates. Avian Pathology 27, 382–389.
Permin, A., Bisgaard, M., Frandsen, F., Pearman, M., Nansen, P., Kold, J., 1999. The prevalence of gastrointestinal helminths in different poultry production systems. Br. Poultry Sci., in press.
Petkevi˘cius, S., Larsen, M., Back Knudsen, K.E., Nansen, P., Grønvold, J., Bjørn, H., 1998. The effect of the nematode-destroying fungus Duddingtonia flagrans against Oesophagostomum dentatum larvae in faeces from pigs fed different diets. Helminthologia 35, 111–116.
Petkevi˘cius, S., Nansen, P., Knudsen, K.E.B., Skjøth, F., 1999. The effect of increasing levels of insoluble dietary fibre on the establishment and persistence of Oesophagostomum dentatum in pigs. Parasite. 6, 17–26. Pozio, E., 1998. Trichinellosis in the European Union: epidemiology, ecology, ecology and economic impact.
Parasitol. Today 14, 35–38.
Roepstorff, A., Murrell, K.D., 1997. Transmission dynamics of helminth parasites of pigs on continuous pasture:
Ascaris suum and Trichuris suis. Int. J. Parasitol. 27, 563–572.
Roepstorff, A., Nilsson, O., 1991. En fællesnordisk prævalensundersøgelse. In: Eriksen, L., Roepstorff, A., Nansen, P. (Eds.), Parasitære infektioner hos svin. NKJ projekt 59. København. pp. 16–53.
Roepstorff, A., Jørgensen, R.J., Nansen, P., Henriksen, S.A., Skovgaard Pedersen, J., Andreasen, M., 1992. Parasitter hos økologiske svin. Rapport over projekt finansieret af Jordbrugs—direktoratet under Landbrugsministeriet. Landsudvalget for svin, Danske Slagterier. 36 pp.
Roepstorff, A., Nilsson, O., Oksanen, A., Gjerde, B., Richter, S., Örtenberg, E., Christensson, D., Martinsson, K.B., Bartlett, P.C., Nansen, P., Eriksen, L., Helle, O., Nikander, S., Larsen, K., 1998. Intestinal parasites in swine in the Nordic countries: prevalences and geographical distribution. Vet. Parasitol. 76, 305–319. Shaw, D.J., Vercruysse, J., Claerebout, E., Dorny, P., 1998. Gastrointestinal nematode infections of first-grazing
season calves in Western Europe: associations between parasitological, physiological, physiological and physical factors. Vet. Parasitol. 75, 133–151.
Smith, H.J., 1979. Transmission of Oesophagostomum species in swine on pasture in the maritime provinces. Can. Vet. J.20, 184–185.
Thamsborg, S.M., Jørgensen, R.J., Waller, P.J., Nansen, P., 1996. The influence of stocking rate on gastrointestinal nematode infections of sheep over a two-year grazing period. Vet. Parasitol. 67, 207–224.
Thamsborg, S.M., Jørgensen, R.J., Nansen, P., 1998a. Internal parasitism of steers grazing at different stocking rates. Acta Vet. Scand. 39, 311–323.
Thamsborg, S.M., Jørgensen, R.J., Ranvig, H., Waller, P.J., Nansen, P., 1998b. The performance of grazing sheep in relation to stocking rate and exposure to nematode infections. Livest. Prod. Sci. 53, 265–277.
Vaarst, M., Thamsborg, S.M., Nematode infections in organic dairy cattle herds in Denmark. Proc. Baltic-Scandinavian Symposium on Parasitic Zoonoses and Ecology of Parasites, Vilnius, Lithuania, 7–8 Sept.1994. Bull. Scand. Soc. Parasitol. 5, 54–55
Vaarst, M., Ploeger, H., Thamsborg, S.M., Sørensen, J.T., 1996. Organic dairy farming and nematode parasitism. Infection patterns among replacement heifers in dairy herds not preventively treated wuth antihelmintics. In: Dent, Mc. Gregor, Sibbald (Eds.), Livestock Farming Systems. EAAP Publication No. 79,Wageningen Pers, pp. 85–90.
Waghorn, G.C., Reed, J.D., Ndlovu, L.R., 1997. Condensed tannins and herbivore nutrition. Proc. 18th Int. Grasslands Congress, Winnipeg and Saskatoon, in press.