H YPOSTHENURIAin patients with sickle cell anemia was first reported by Josephs.' It was soon established that the defect in urinary concentration was not due to a deficiency of antidiuretic hormone and therefore a renal pathogenosis was sug gested.2'3 Zarafonetis et al.@ first reported that subjects with the sickle cell trait also failed to concentrate urine normally and postulated a genetic basis for the hypos thenuria. However, Keitel et al.5 demon strated that the hyposthenuria of young pa tients with sickle cell anemia was reversi ble and indicated that renal damage due to intravascular sickling or the presence of the sickle-cell type of hemoglobin may result in renal dysfunction and hyposthenuria.
In the present report the maxima for urinary concentration were determined in patients with sickle cell anemia and in sub jects with the sickle cell trait of different ages to further clarify the pathogenesis of this hyposthenuria.
SUBJECTS
Twenty-three subjects with the sickle cell trait and 14 patients with sickle cell anemia were investigated. Those with the sickle cell trait showed positive sickling with the bisulfite methodo but less than 50% S-hemoglobin by paper electrophoresis.7 The patients with sickle cell anemia showed positive sickling with the bisulfite method and more than 50% 5-hemoglobin by paper electrophoresis. Some of the patients with sickle cell anemia had re ceived blood transfusion on previous admis sions, but not within 6 months of the renal studies. Patients 5, 8 and 12 had sickle cell C-hemoglobin disease.
METHODS
A routine concentration test was used to
determine the maximal ability to concentrate urine. Food and water were withheld for 12 or more hours before the test. Two hours be fore the test, the bladder was emptied. At the beginning of the test a urine sample was col lected, following which 0.1 ml, 2 units, of vasopressin (Pitressin®) was administered sub cutaneously. A second dose of vasopressin was given 30 minutes later. The second urine sample was collected 60 minutes following the first injection of vasopressin. The urine sample collected after the administration of vasopres sin was usually slightly more concentrated than the initial urine sample, but if a marked in crease in osmolar concentration occurred, the test was considered to be unsatisfactory. This would indicate that the patient may not have thirsted for 12 or more hours; in these pa tients the test was repeated. Urine samples from infants under 2 to 3 years of age were obtained by catheter. Except for hyposthe nuria, evidence of renal disease was not pres ent, as determined by history, physical or laboratory examinations or both. Hematuria was not known to have been present, and routine urinalyses were normal except for low values for specific gravity.
The osmolar concentration of urine was determined with a Bowman freezing-point apparatus.6 The accuracy attained was within 2%. The urine concentrations are expressed in mosml/l (milliosmoles/l). One mosm.l is equiv alent to a solute concentration of one milli mole of “¿ideal―solute dissolved in 1 kg of water.
RESULTS
The urinary findings to be reported are compared to the maxima of urinary con centration of 33 normal controls (reported previously5) who had a mean value of 1,055 mosml/l with a standard deviation of 118. Neither age nor race were found to in fluence the maxima of urine concentration Supported by a grant (H3282) from the National Institutes of Health.
ADDRESS:(H.G.K.) 1025 Walnut Street, Philadelphia 7, Pennsylvania.
PEDIATRICS, August 1960
249
PATHOGENESISOF HYPOSTHENURIAIN PERSONSWITH
SICKLECELL ANEMIA OR THE SICKLECELLTRAIT
L. Schlitt, M.D., and H. G. Keitel, M.D.
Pt.SexAge
(yr)Hemoglobin(gm/100Maximum Urinary Concentration
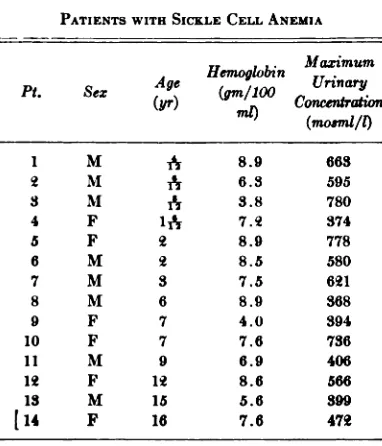
(mosmi/l)1M,@8.9663M6.35953M3.87804F1@7.@3745F8.97786M8.55807M37.56@18M68.93689F74.039410F77.673611M96.94061@F1@8.656613M155.6399[14F167.647@
250 SICKLE CELL ANEMIA
mOemL 1200
I000
800
600
400
200
@////////////////////////t/////// 0
. S. 0
S 0
. S S S
•¿â€¢¿
. S
S .
0 0
0
00
•¿Sickle cell anemia
oSicklecell frelt
@Norrvi@lSubjects
0 0
S I •¿ S S •¿
S •¿ S
- S
0 0
:
S.2 4 6 8 10 20 30 40
AGE IN YEARS
Fic. 1. The maxima of urinary concentration (mosml/l) in the subjects as indicated. (See
text for comment.)
in the normal subjects, all of whom were under 38 years of age (Fig. 1).
The results of the urinary studies of pa tients with sickle cell anemia are listed in
TABLE I
PATIENTS WITH SICKLE CELL ANEMIA
Table I and the findings in subjects with the sickle cell trait are listed in Table II. Figure 1 shows the maximum solute concen tration in urine following the standard test in patients with sickle cell anemia and in subjects with sickle cell trait of different ages. The Figure includes 14 patients with sickle cell anemia not previously reported (Table I) and 29 patients with sickle cell anemia reported previously.5 Figure 1 also includes 23 subjects with sickle cell trait not previously reported (Table II) and 26 subjects with sickle cell trait reported previ ously.5 The distribution of the maxima of urinary concentration is such that a line drawn from 800 mosmi at birth to 300 mosmi at 50 years of age almost completely separates the patients with sickle cell anemia from subjects with the sickle cell trait (Fig. 1). Five subjects with sickle cell trait are just below this line and two pa tients with sickle cell anemia are just above
the line.
MaximumPt.SexAge
(yr)Ilemo
.
globln
(gm/100 ml)Urinary Concen
tration(mosml/l)IM2@10.29682F312.29003M10.48204F3@11.59465M12.79906M3f-@12.49987F4@'@12.57208M4@410.28219M4@11.585110M4@-@-12.162811F59.455212i@L5@—94413F5@10.61,04414F7-@12.666315M812.858316F811.774217F81169018M912.870219M1012.270620M111354421F1213.384422M1311.980623F1711.5824 TABLE II
SUBJECTS WITH SICKLE CELL TRAIT
I5Occ RBC
1000
000
m@mI
600
400
I 3 5 7 9 II 3 5 7 9 21 23
DAYS AFTER ADMISSION
Ftc. 2. The maxima of urinary concentration (mosml/l) in subject H. J., 6 months of age, with sickle cell anemia, before and after transfusion with
packed normal erythrocytes.
age. Following two transfusions of normal erythrocytes, his maximum urinary concen tration rose to 1020 mosml (Fig. 2). This patient represents the fifth recorded in stance of complete reversibility of hypos
thenuria in patients with sickle cell anemia following the transfusion of normal erythro cytes in young patients.5
The subjects with sickle cell trait as a group were also found to have hyposthe nuria. The maxima of urinary concentration for the subjects with sickle cell trait are
significantly lower than those of the con
trol group (753 vs. 1,055 mosml), with a
P(t) value of less than 0.001 (Fig. 3). The
maxima of urinary concentration of the group with sickle cell trait is more variable than in the control group (variance rate F is less than 0.05). Of 15 subjects with sickle range in which the values fall is wide dur
ing the early years of life, when an occa sional value in the third standard deviation of the normal is seen, but is narrow after
the tenth year of life. Only one patient with sickle cell anemia over the age of 7 years (Patient 12 with sickle cell C-hemoglobin
disease) was able to concentrate urine over
500 mosml, whereas a half of the patients with sickle cell anemia under 7 years of age
had maxima for urinary concentration over
500 mosml. Four of the patients in the
younger age group concentrated the urine to over 700 mosml. There is a statistically
significant, inverse correlation between the maxima of urinary concentration and in creasing age; P(t) is less than 0.001 and the Spearman coefficient of rank correlation is (P = —¿0.52).
Patient 2 with sickle cell anemia con centrated urine after the standard vaso pressin test to 595 mosml at 6 months of
40
0 .!. 30
@0 3
@.- 20
0
I0
400 550 700 051 000 ISO 300
mosml/l
Fic. 3. Distribution curves of maxima of urinary concentration for control subjects (right) and for
patients with sickle cell anemia (left).
p1-.
I//il @
@ @ @
@
@ “¿@23
252 SICKLE CELL ANEMIA cell trait under 6 years of age, 80% had
maxima of urinary concentration which were in the normal range; but out of 34 subjects with sickle cell trait over 6 years of age, only 29% concentrated urine norm ally. With advancing age, the subjects with sickle cell trait were found to have both normal and impaired maxima of urinary concentration. Taken as a group, there is a statistically significant inverse correlation between the maxima of urinary concentra tion and increasing age; P(t) is less than 0.05 and the Spearman coefficient of rank correlation is (P —¿0.33).
DISCUSSION
From these data one may conclude that the defect in urinary concentration of sub jects whose erythrocytes contain S-hemo globin may be influenced by at least two factors: 1) the amount of S-hemoglobin in erythrocytes; and 2) the age of the subject. The degree of anemia per se is not believed to influence the findings.5 Since the amount of S-hemoglobin remains relatively con stant after the first year of life, the first fac tor is not responsible for the second, at least after the first year of life. Subjects with sickle cell trait, who have a lower percent age of S-hemoglobin in erythrocytes than do patients with sickle cell anemia, have less hyposthenuria than patients with sickle cell anemia.
Four patients with sickle cell C-hemo globin disease are included in the group with sickle cell disease. The maxima of urinary concentration in three of these pa tients were found to be in the upper level of the range of data obtained in patients with sickle cell anemia, but one patient with sickle cell C-hemoglobin disease had a lower urine concentration than patients with sickle cell anemia of comparable age. The number of observations in patients with sickle cell C-hemoglobin disease is too small to suggest that the defect in urine concentration is less than in patients with sickle cell disease.
As subjects with sickle cell trait get older, hyposthenuria becomes more pronounced.
Hyposthenuria also has been reported to occur in aged “¿normal―subjects.9 Why some patients with sickle cell anemia exhibit the defect in urinary concentration earlier than others, and why some patients with sickle cell trait completely escape this defect,
cannot be explained. From previously re
ported data5 it would appear that the se verity of hyposthenuria is not related to the amount of fetal (F) hemoglobin in erythro cytes.
The reversibility of hyposthenuria follow ing the administration of normal erythro
cytes appears to make a genetic basis for
the renal defect unlikely.
The amount of urea excreted affects the concentration of urine, but this is not be lieved to be an etiologic factor in the hy posthenuria of patients with sickle cell trait or sickle cell disease because their rate of excretion of urea is normal.b0 Furthermore, the rapid reversibility of hyposthenuria and the progression of hyposthenuria with age could not easily be explained by a change in urea metabolism.
An abnormality of renal hemodynamics (glomerular filtration rate, renal blood flow) also does not seem to be a reasonable ex planation for the hyposthenuria.5h1 How ever, since hydrostatic pressure and electro lyte transport in the medullary and papil lary areas of the kidney cannot be meas ured by present techniques, it is possible that alterations of these factors are present in subjects with sickle cells. Cellular pa thology is present in these areas of the kidney.
to concentrate urine. Necropsy reports of subjects with sickle cell trait for whom electrophoretic proof of the diagnosis was available, are scarce.
Chapman et al.13 report one case of proven sickle cell trait with normal renal parenchyma being found at nephrectomy. Mostofi et
@ report 21 cases of nephrec
tomy or renal biopsy in subjects with the clinical diagnosis of sickle cell trait and the finding of stasis of sickled erythrocytes in small vessels, principally in the medulla and the papillae, with focal hemorrhages in the interstitium, in the tubules or in the pelvis or both. In about a half of these pa tients, epithelial degeneration, proliferation and necrosis were found.
Clinical evidence that subjects with sic kle cell trait are sometimes adversely af fected by their hemoglobin abnormality is well documented.15,
@ Hypoxia simulating
an altitude of 7,000 to 8,000 feet produced shortening of the erythrocyte survival-time in subjects with sickle cell trait, and high altitude flying precipitated sickle cell crisis in patients with sickle cell trait.'7'― Pain less, unilateral hematuria also has been re ported in subjects with sickle cell trait.―'°
It seems reasonable to assume that the defect in renal concentration by subjects with sickle cell trait as well as by patients with sickle cell anemia is due to an altera tion in tubular function caused by clinical or subclinical sickling of erythrocytes. Tubular damage due to the presence of circulating S-hemoglobin, which is present in low concentration in the plasma of pa tients with the trait and in higher concen tration in the plasma of patients with the anemia,2' cannot be excluded as an addi tional possible cause of hyposthenuria.
SUMMARY
Hyposthenuria was investigated in sub jects with sickle cell trait and in patients with sickle cell anemia. The following were observed: 1) in subjects with sickle cell trait both normal and reduced maxima of un nary concentration are found, whereas all untreated patients with sickle cell anemia
over 6 months of age have hyposthenunia; 2) hyposthenuria becomes increasingly more severe with advancing age in both sickle cell anemia and sickle cell trait; 3) in a 6-month-old patient with sickle cell anemia and hyposthenuria, the maxima of urinary concentration returned to normal after two transfusions of normal erythrocytes.
Reasons are presented for favoring the hypothesis that hyposthenunia in sickle cell disease is due to renal damage, possibly from intravascular sickling of erythrocytes in renal vessels or from the presence of “¿free―circulating S-hemoglobin.
Acknowledgment
The authors acknowledge the assistance of Dr. Menduke of the Department of Biosta tistics, Jefferson Medical College, and of Dr. Felix E. Karpinski for referring patients for study.
REFERENCES
1. Josephs, H.: Clinical aspects of sickle cell anemia. Bull. Johns Hopkins Hosp., 43: 397, 1928.
2. McCrory, W. W., Goren, N., and Corn feld, D.: Demonstration of impairment of urinary concentration ability or pitres sin resistance in children with sickle cell anemia. Am. J. Dis. Child., 85:512, 1953.
3. Kunz, H. W., Pratt, E. L., Mellin, G. W., and Chenng, M. W.: Impairment of urinary concentration in sickle cell ane mia. PEDIATRICS,13:352, 1954. 4. Zarafonetis, C. J. C., Steiger, W. A., Hol
than, L., McMaster, j., and Colville, V. F.: Renal defect associated with sickle cell trait and sickle cell disease.
J. Lab. & Clin. Med.,44:959, 1954.
5. Keitel, H. G., Thompson, D., and Itano, H. C.: Hyposthenuria in sickle cell ane mia—a reversible renal defect. J. Clin. Invest., 35:998, 1956.
6. Daland, C. H., and Castle, W. B.: A sim ple and rapid method for demonstrating sickling of the red blood cells—the use of reducing agents. J. Lab. & Clin. Med., 33:1082, 1948.
7. Goldberg, C. A. J.: Identification of human hemoglobins. Clin. Chem., 3:1, 1957. 8. Bowman, R. L., Tranthanm, H. V., and
254 SICKLE CELL ANEMIA Lab. & Clin. Med., 43:310, 1954.
9. Miller, J. H., and Shock, N. W.: Age dif ferences in the renal tubular response to antidiuretic hormone. J. Cerontol., 8: 446, 1953.
10. Keitel, H. C., and Schlitt, L. E.: Unpub lished data.
11. Levitt, M. F., Marshall, S. L., Hanser, A. D., and Polimeros, D.: The concentrat ing defect in sickle cell disease (Ab stract). A.M.A. J. Dis. Child., 98:616, 1959.
12. Bernstein, J., and Whitten, C. F.: A histo logic appraisal of the kidney in sickle cell anemia (Abstract). A.M.A. J. Dis. Child., 98:562, 1959.
13. Chapman, A. F., Reeder, P. S., Friedman,
J. A.,and Baker,L. A.: Crosshematuria
in sickle cell trait and sickle cell-hemo globin C disease. Am. J. Med., 19:773,
1955.
14. Mostofi, F. K., Vorder Bruegge, C. F., and Diggs, L. W.: Lesions in kidneys re moved for unilateral hematuria in sickle cell disease. Arch. Path., 63:336, 1957. 15. Sproule, B. J., Halden, E. R., and Miller, W. F.: A study of cardiopulmonary al terations in patients with sickle cell dis
ease and its variants. J. Clin. Invest., 37: 486, 1958.
16. Levin, W. C., Eggers, C. W. N., and Perry,
J. E.: The effectof hypoxiain patients
with sickle cell trait. Clin. Res., 6:190, 1958.
17. Sullivan, B. H., Jr.: Danger of airplane flight to persons with sicklemia. Ann.
mt. Med.,32:338,1950.
18. Coolev, J. C., Peterson, W. L., Engel, C. E,. and Jernigan, J. P.: Clinical triad of massive splenic infarction, sicklemia trait, and high altitude flying. J.A.M.A., 154:111, 1954.
19. Abel, M. S., and Brown, C. R.: Sickle cell disease with severe hematuria simulat ing renal neoplasm. J.A.M.A., 136:624, 1948.
20. Goodwin, W. E., Alston, E. F., and Se mans, J. H.: Hematuria and sickle cell disease—unexplained, gross unilateral, renal hematuria in Negroes, coincident with the blood sickling trait. J. Urol., 63:79, 1950.
1960;26;249
Pediatrics
L. Schlitt and H. G. Keitel
ANEMIA OR THE SICKLE CELL TRAIT
PATHOGENESIS OF HYPOSTHENURIA IN PERSONS WITH SICKLE CELL
Services
Updated Information &
http://pediatrics.aappublications.org/content/26/2/249 including high resolution figures, can be found at:
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or in its
Reprints
1960;26;249
Pediatrics
L. Schlitt and H. G. Keitel
ANEMIA OR THE SICKLE CELL TRAIT
PATHOGENESIS OF HYPOSTHENURIA IN PERSONS WITH SICKLE CELL
http://pediatrics.aappublications.org/content/26/2/249
the World Wide Web at:
The online version of this article, along with updated information and services, is located on
American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.