inorganic papers
i70
Ivashkevichet al. LiCrP2O7 doi:10.1107/S1600536807005752 Acta Cryst.(2007). E63, i70–i72
Acta Crystallographica Section E
Structure Reports Online
ISSN 1600-5368
X-ray powder diffraction study of LiCrP
2O
7Ludmila S. Ivashkevich,* Kirill A. Selevich, Anatoly I. Lesnikovich and Anatoly F. Selevich
Research Institute for Physico-Chemical Problems, Belarusian State University, Leningradskaya Street 14, Minsk 220030, Belarus
Correspondence e-mail: iva@bsu.by
Key indicators
Powder X-ray study T= 295 K
Mean(P–O) = 0.015 A˚ Rfactor = 0.029 wRfactor = 0.038
Data-to-parameter ratio = 6.02
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 31 January 2007 Accepted 2 February 2007
#2007 International Union of Crystallography All rights reserved
The monoclinic crystal structure of lithium chromium(III) diphosphate, LiCrP2O7, isotypic with other members of the
series LiMIIIP2O7 (M III
= Mn, Fe, V, Mo, Sc and In), was refined from laboratory X-ray powder diffraction data using the Rietveld method. The Cr3+cation is bonded to six O atoms from five diphosphate anions to form a distorted octahedron. Links between the bent diphosphate anions and the Cr3+ cations result in a three-dimensional network, with tunnels filled by the Li+cations in a considerably distorted tetrahedral environment of O atoms.
Comment
Double diphosphates LiMIIIP2O7(M III
is a trivalent metal) are of interest because of their ionic conductivity at high temperatures (Vıtinnsˇ et al., 2000). The crystal structures of LiMIIIP2O7compounds have already been described forM
III
= In (Tran Quiet al., 1987), Fe (Riouet al., 1990), Mo (Ledainet al., 1996), Sc (Vıtinnsˇ et al., 2000), V (Rousseet al., 2001) and Mn (Ivashkevichet al., 2006). All these compounds crystallize in the monoclinic space groupP21and are isotypic. Here, we
report the crystal structure of the CrIIImember of this series, LiCrP2O7, (I), refined from laboratory X-ray powder
diffrac-tion data.
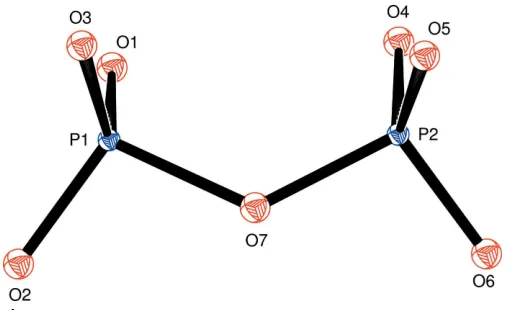
The asymmetric unit of (I) comprises one Li, one Cr, two P and seven O atoms, all in general positions. The conformation of the diphosphate anion is close to eclipsed and exhibits a bent P—O—P angle of 127.2 (5). The average of the dihedral
angles O1—P1 P2—O4, O2—P1 P2—O6 and O3—
P1 P2—O5 is 9.1 (Figs. 1 and 2). All other distances and
[image:1.610.206.459.559.714.2]angles are in the usual ranges observed for many other diphosphates (Durif, 1995).
Figure 1
The Cr atoms have a distorted octahedral environment of O atoms from five diphosphate groups, with an average Cr—O distance of 1.973 A˚ . The Cr3+cations connect the diphosphate anions to form a three-dimensional network, with channels filled by Li+cations (Fig. 3). The Li+cations have a strongly distorted tetrahedral environment of four O atoms from four diphosphate anions (Table 1), with one O—Li—O angle of 175.8 (12) significantly different from the ideal tetrahedral
angle.
A detailed comparison of (I) with the isotypic structures of the LiMIIIP2O7diphosphates (MIII = Mn, Fe, V, Mo, Sc, In)
shows that the geometric features of the diphosphate anions (terminal and bridging P—O bond lengths, P—O—P and O— P—O angles, and torsion angles) and of the LiO4polyhedron
are quite similar in all structures. In the isotypic series, there is a monotonic increase of the unit-cell volume (A˚3) in the following sequence of MIII salts: 249.32 (Cr), 254.77 (Mn), 255.22 (Fe), 256.02 (V), 272.85 (Mo), 274.05 (Sc) and 274.28 (In), which is in accordance with the increase of the ionic radii (Shannon, 1976) from Cr3+ to In3+, provided that Mn3+ and Fe3+ are in their high-spin state (otherwise LiMnP2O7 and
LiFeP2O7 would have the smallest cell volumes). The
assumption of the presence of Fe3+ high-spin cations in LiFeP2O7 has been confirmed by magnetic measurements
(Whangboet al., 2004).
Experimental
The title compound was prepared by the technique described previously by Selevich et al. (2006). A mixture of CrCl36H2O
(2.40 g), Li2CO3(0.33 g) and (NH4)2HPO4(2.38 g) was placed in a
platinum crucible and heated to 1173 K at a rate of 5 K min1. After
cooling to room temperature, washing with water and drying at 373 K, a colourless microcrystalline solid was obtained. The compound was further identified by chemical analysis and quanti-tative thin-layer chromatography.
Crystal data
LiCrP2O7
Mr= 232.88
Monoclinic,P21
a= 6.9019 (10) A˚
b= 7.9919 (11) A˚
c= 4.7807 (7) A˚
= 109.003 (4) V= 249.32 (6) A˚3
Z= 2
Dx= 3.102 Mg m 3
CuKradiation
= 1.5418 A˚
T= 295 (2) K
Specimen shape: flat sheet 30301 mm
Particle morphology: no specific habit,, colourless
inorganic papers
Acta Cryst.(2007). E63, i70–i72 Ivashkevichet al. LiCrP
[image:2.610.313.564.73.270.2] [image:2.610.82.267.74.244.2]2O7
i71
Figure 2
The conformation of the diphosphate anion in (I), viewed approximately along the P1 P2 backbone. Displacement ellipsoids are drawn at the 50% probability level.
Figure 3
[image:2.610.45.294.296.559.2]The crystal structure of (I), viewed along thecaxis. The atom-numbering scheme is shown for the asymmetric unit. Displacement ellipsoids are drawn at the 50% probability level.
Figure 4
Final plots of the Rietveld refinement, showing the experimental (circles) and calculated (line) intensities of (I) in the range 2= 12–72. The
Data collection
Carl Zeiss HZG-4A diffractometer Specimen mounting: packed powder
pellet
Specimen mounted in reflection mode
Scan method: step 2min= 12.0
, 2max= 100
Increment in 2= 0.02
Excluded region(s): none
Refinement
Refinement onInet
Rp= 0.029
Rwp= 0.038
Rexp= 0.101
RB= 0.064
S= 0.35
Profile function: pseudo-Voigt profile function,= 0.61(6)
307 reflections 51 parameters
w= 1/(Yobs)
(/)max= 0.02
Preferred orientation correction: none
Table 1
Selected geometric parameters (A˚ ,).
Cr—O1 1.99 (2) Cr—O4 1.996 (19) Cr—O3i 1.91 (3) Cr—O6ii 2.040 (19) Cr—O5iii 1.980 (16) Cr—O2iv 1.924 (11) P1—O1 1.529 (17) P1—O2 1.542 (11) P1—O3 1.53 (2)
P1—O7 1.629 (12) P2—O4 1.487 (8) P2—O5 1.55 (2) P2—O6 1.499 (17) P2—O7 1.611 (12) Li—O2 2.102 (12) Li—O4v 2.049 (11) Li—O6iv
1.912 (16) Li—O5vi
1.966 (17)
O1—P1—O2 110.8 (12) O1—P1—O3 112.8 (12) O1—P1—O7 107.6 (9) O2—P1—O3 115.2 (12) O2—P1—O7 101.4 (6) O3—P1—O7 108.1 (11) O4—P2—O5 110.1 (8) O4—P2—O6 114.1 (8) O4—P2—O7 108.1 (8)
O5—P2—O6 113.5 (7) O5—P2—O7 111.0 (9) O6—P2—O7 99.5 (7) O2—Li—O4v 92.6 (8) O2—Li—O5vi 76.9 (12) O2—Li—O6iv 175.8 (12) O4v—Li—O5vi 107.7 (11) O4v —Li—O6iv 83.3 (10) O5vi —Li—O6iv 104.0 (8)
Symmetry codes: (i) x;y;z1; (ii) x;y1
2;z; (iii) x;y 1
2;zþ1; (iv)
xþ1;y1
2;zþ1; (v)xþ1;y;zþ1; (vi)xþ1;y;z.
The diffraction pattern of (I) could be indexed with a monoclinic cell and reasonable reliability factors (M20= 43,F20= 54,M40= 26,F40
= 35) using the programTREOR90(Werneret al., 1985). The unit-cell dimensions obtained indicated isotypism with compounds of the type LiMIIIP2O7 that crystallize in the monoclinic space group P21.
Therefore, this space group and the atomic coordinates of LiMnP2O7
(Ivashkevichet al., 2006) were used as starting parameters for the refinement of the structure using the Rietveld method as imple-mented in the FULLPROF program (Rodrı´guez-Carvajal, 2001). Because space groupP21is polar, theycoordinate of the Cr atom was
fixed. A correction for profile asymmetry was made for reflections up to 2= 40. In view of an unstable refinement of the displacement
parameters of some atoms, they were fixed with values similar to those for other isotypic compounds [Uiso(Cr, P) = 0.005,Uiso(O) =
0.0095 andUiso(Li) = 0.025 A˚
2]. No preferred orientation of grains in
the sample was found. During structure refinement, soft restraints for the interatomic distances of the diphosphate group based on a geometric analysis of a large number of diphosphates (Durif, 1995), and also for Li—O distances, were used. Fig. 4 shows the observed and calculated diffraction patterns of (I) for the final refinement.
Data collection: local program; cell refinement: FULLPROF
(Rodrı´guez-Carvajal, 2001); data reduction: local program; method used to solve structure: coordinates taken from an isotypic compound (Ivashkevich et al., 2006); program(s) used to refine structure:
FULLPROF; molecular graphics:ORTEP-3 for Windows(Farrugia, 1997); software used to prepare material for publication: FULL-PROFandPLATON(Spek, 2003).
References
Durif, A. (1995).Crystal Chemistry of Condensed Phosphates, pp. 73–77. New York: Plenum Press.
Farrugia, L. J. (1997).J. Appl. Cryst.30, 565.
Ivashkevich, L. S., Selevich, K. A., Lesnikovich, A. I., Selevich, A. F. & Lyakhov, A. S. (2006).Z. Kristallogr.221, 115–121.
Ledain, S., Leclaire, A., Borel, M. M. & Raveau, B. (1996).Acta Cryst.C52, 1593–1594.
Riou, D., Nguyen, N., Benloucif, R. & Raveau, B. (1990).Mater. Res. Bull.25, 1363–1369.
Rodrı´guez-Carvajal, J. (2001). FULLPROF. Version 1.9c. CEA/Saclay, France.
Rousse, G., Wurm, C., Morcrette, M., Rodriguez-Carvajal, J., Gaubicher, J. & Masquelier, C. (2001).Int. J. Inorg. Mater.3, 881–887.
Selevich, K. A., Lesnikovich, A. I., Grushevich, E. V. & Selevich, A. F. (2006). Belarussian Patent Appl. A20061215.
Shannon, R. D. (1976).Acta Cryst.A32, 751–767. Spek, A. L. (2003).J. Appl. Cryst.36, 7–13.
Tran Qui, D., Hamdoune, S. & Le Page, Y. (1987).Acta Cryst.C43, 201–202. Vıtinnsˇ, G., Kanepe, Z., Vı tinnsˇ, A., Ronis, J., Dindu ne, A. & Lusis, A. (2000).J.
Solid State Electrochem.4, 146–152.
Werner, P.-E., Eriksson, L. & Westdahl, M. (1985).J. Appl. Cryst.18, 367–370. Whangbo, M.-H., Dai, D. & Koo, H.-J. (2004).J. Chem. Soc. Dalton Trans.pp.
3019–3025.
inorganic papers
i72
Ivashkevichet al. LiCrPsupporting information
sup-1 Acta Cryst. (2007). E63, i70–i72
supporting information
Acta Cryst. (2007). E63, i70–i72 [https://doi.org/10.1107/S1600536807005752]
X-ray powder diffraction study of LiCrP
2O
7Ludmila S. Ivashkevich, Kirill A. Selevich, Anatoly I. Lesnikovich and Anatoly F. Selevich
Lithium chromium(III) diphosphate(V)
Crystal data
LiCrP2O7
Mr = 232.88
Monoclinic, P21 Hall symbol: P 2yb
a = 6.9019 (10) Å
b = 7.9919 (11) Å
c = 4.7807 (7) Å
β = 109.003 (4)°
V = 249.32 (6) Å3
Z = 2
Dx = 3.102 Mg m−3
Cu Kα radiation, λ = 1.54180 Å
T = 295 K colourless
flat_sheet, 30 × 30 mm
Specimen preparation: Prepared at 295 K
Data collection
Carl Zeiss HZG-4A diffractometer
Radiation source: fine-focus sealed X-ray tube, BSV-29
Specimen mounting: packed powder pellet Data collection mode: reflection
Scan method: step
2θmin = 12.00°, 2θmax = 100.00°, 2θstep = 0.02°
Refinement
Refinement on Inet
Least-squares matrix: full with fixed elements per cycle
Rp = 0.029
Rwp = 0.038
Rexp = 0.101
RBragg = 0.064
Excluded region(s): none
Profile function: pseudo-Voigt profile function,
η = 0.61(6)
51 parameters 12 restraints 0 constraints
Weighting scheme based on measured s.u.'s w = 1/σ(Yobs)
(Δ/σ)max = 0.02
Background function: Polynomial background function, with six polynomial coefficients refined
Preferred orientation correction: none
Special details
Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Cr 0.2277 (13) 0.25000 0.216 (2) 0.0050*
P1 0.4190 (4) 0.4669 (3) 0.7984 (5) 0.0050*
P2 0.0196 (4) 0.5654 (3) 0.4005 (5) 0.0050*
supporting information
sup-2 Acta Cryst. (2007). E63, i70–i72
O2 0.6216 (9) 0.5652 (9) 0.869 (5) 0.0095*
O3 0.351 (4) 0.427 (3) 1.065 (3) 0.0095*
O4 0.006 (3) 0.3996 (9) 0.254 (2) 0.0095*
O6 −0.0177 (16) 0.711 (2) 0.192 (4) 0.0095*
O5 −0.116 (3) 0.5673 (11) 0.602 (4) 0.0095*
O7 0.2562 (9) 0.5994 (9) 0.589 (4) 0.0095*
Li 0.8226 (9) 0.3780 (6) 0.8219 (11) 0.0250*
Geometric parameters (Å, º)
Cr—O1 1.99 (2) P1—O7 1.629 (12)
Cr—O4 1.996 (19) P2—O4 1.487 (8)
Cr—O3i 1.91 (3) P2—O5 1.55 (2)
Cr—O6ii 2.040 (19) P2—O6 1.499 (17)
Cr—O5iii 1.980 (16) P2—O7 1.611 (12)
Cr—O2iv 1.924 (11) Li—O2 2.102 (12)
P1—O1 1.529 (17) Li—O4v 2.049 (11)
P1—O2 1.542 (11) Li—O6iv 1.912 (16)
P1—O3 1.53 (2) Li—O5vi 1.966 (17)
O1—Cr—O4 93.6 (8) O3—P1—O7 108.1 (11)
O1—Cr—O3i 85.8 (8) O4—P2—O5 110.1 (8)
O1—Cr—O6ii 174.0 (7) O4—P2—O6 114.1 (8)
O1—Cr—O5iii 90.4 (9) O4—P2—O7 108.1 (8)
O1—Cr—O2iv 98.6 (10) O5—P2—O6 113.5 (7)
O3i—Cr—O4 92.3 (9) O5—P2—O7 111.0 (9)
O4—Cr—O6ii 81.5 (6) O6—P2—O7 99.5 (7)
O4—Cr—O5iii 89.0 (7) P1—O7—P2 127.2 (5)
O2iv—Cr—O4 164.1 (7) O2—Li—O4v 92.6 (8)
O3i—Cr—O6ii 91.0 (7) O2—Li—O5vi 76.9 (12)
O3i—Cr—O5iii 176.1 (10) O2—Li—O6iv 175.8 (12)
O2iv—Cr—O3i 98.7 (9) O4v—Li—O5vi 107.7 (11)
O5iii—Cr—O6ii 92.9 (8) O4v—Li—O6iv 83.3 (10)
O2iv—Cr—O6ii 86.9 (8) O5vi—Li—O6iv 104.0 (8)
O2iv—Cr—O5iii 80.9 (6) Cr—O1—P1 128.7 (14)
O1—P1—O2 110.8 (12) Crvii—O2—P1 149.2 (12)
O1—P1—O3 112.8 (12) Crviii—O3—P1 140.0 (13)
O1—P1—O7 107.6 (9) Cr—O4—P2 130.1 (12)
O2—P1—O3 115.2 (12) Crix—O5—P2 133.0 (7)
O2—P1—O7 101.4 (6) Crx—O6—P2 132.0 (10)
O4—Cr—O1—P1 −19.8 (18) O7—P1—O2—Crvii −15 (2)
O3i—Cr—O1—P1 72.2 (18) O1—P1—O3—Crviii −12 (3)
O5iii—Cr—O1—P1 −108.8 (17) O2—P1—O3—Crviii −141 (2)
O2iv—Cr—O1—P1 170.3 (16) O7—P1—O3—Crviii 106 (2)
O1—Cr—O4—P2 34.4 (10) O1—P1—O7—P2 54.3 (16)
O3i—Cr—O4—P2 −51.6 (10) O2—P1—O7—P2 170.7 (12)
supporting information
sup-3 Acta Cryst. (2007). E63, i70–i72
O5iii—Cr—O4—P2 124.7 (9) O5—P2—O4—Cr −131.8 (11)
O1—Cr—O3i—P1i 141 (2) O6—P2—O4—Cr 99.3 (9)
O4—Cr—O3i—P1i −126 (2) O7—P2—O4—Cr −10.3 (11)
O4—Cr—O6ii—P2ii −152.9 (12) O4—P2—O5—Crix −162.7 (14)
O1—Cr—O5iii—P2iii 71.2 (17) O6—P2—O5—Crix −33.4 (19)
O4—Cr—O5iii—P2iii −22.4 (16) O7—P2—O5—Crix 77.6 (16)
O1—Cr—O2iv—P1iv −15 (2) O4—P2—O6—Crx 42.1 (15)
O2—P1—O1—Cr −122.9 (17) O5—P2—O6—Crx −85.1 (14)
O3—P1—O1—Cr 106.3 (17) O7—P2—O6—Crx 156.9 (11)
O7—P1—O1—Cr −12.8 (19) O4—P2—O7—P1 −43.1 (13)
O1—P1—O2—Crvii 99.6 (19) O5—P2—O7—P1 77.8 (11)
O3—P1—O2—Crvii −131 (2) O6—P2—O7—P1 −162.4 (12)
Symmetry codes: (i) x, y, z−1; (ii) −x, y−1/2, −z; (iii) −x, y−1/2, −z+1; (iv) −x+1, y−1/2, −z+1; (v) x+1, y, z+1; (vi) x+1, y, z; (vii) −x+1, y+1/2, −z+1; (viii)