ARTICLE
Goitrous Autoimmune Thyroiditis in a Pediatric
Population: A Longitudinal Study
Sripathy Gopalakrishnan, MDa, Pradeep Kumar Chugh, MDa, Mitrabasu Chhillar, MDa, Vinod Kumar Ambardar, MSca, Maheshwar Sahoo, MDa,
Rajan Sankar, MDb
aInstitute of Nuclear Medicine and Allied Sciences, Delhi, India;bGlobal Alliance for Improved Nutrition, New Delhi, India
The authors have indicated they have no financial relationships relevant to this article to disclose.
What’s Known on This Subject
Children with AIT can present with euthyroidism, SCH, or even overt hypothyroidism or, rarely, hyperthyroidism. The proportion of thyroid dysfunction at presentation and the natural history of progression vary among different studies.
What This Study Adds
The majority of subjects with goitrous AIT presented with SCH or overt thyroid dysfunc-tion in our study. Because development of thyroid dysfuncdysfunc-tion could be insidious and may not be accompanied by symptoms and signs in children, it is imperative to monitor and correct thyroid dysfunction.
ABSTRACT
BACKGROUND.Patients with autoimmune thyroiditis can present with thyroid function that varies from euthyroidism to frank hypothyroidism or occasionally hyperthy-roidism. Although there is a risk of progression from the euthyroid or subclinical hypothyroid state to frank hypothyroidism, the rate of progression is not known.
OBJECTIVES.Subjects with diffuse goiter and autoimmune thyroiditis were followed up to observe the rate of deterioration in thyroid function from euthyroid and subclin-ical hypothyroid states to hypothyroidism.
METHODS.Patients who presented with goiter and autoimmune thyroiditis were grouped as those with euthyroidism, subclinical hypothyroidism, and overt hypo-thyroidism on the basis of levels of thyroxine and thyrotropin at presentation. Patients were followed up for a minimum duration of 24 months with periodic monitoring of thyroid function.
RESULTS.Ninety-eight consecutive subjects (aged of 8 –18 years) with a diagnosis of autoimmune thyroiditis and diffuse goiter were studied. At presentation, in 24 subjects (24.5%) thyroid function was normal (euthyroidism), 32 (32.6%) had subclinical hypothyroidism, and the remaining 42 subjects (42.9%) had hypothy-roidism. All of the subjects with hypothyroid were maintained euthyroid on thyrox-ine during follow-up. Hypothyroidism developed in 3 of 24 patients with euthyroid-ism and in 4 of 32 patients with subclinical hypothyroideuthyroid-ism.
CONCLUSIONS.Subjects with goitrous autoimmune thyroiditis need periodic monitoring
of thyroid function. Development of thyroid dysfunction is insidious and may not be accompanied by symptoms and clinical signs. In pediatric and adolescent age groups it is imperative to correct thyroid dysfunction to achieve optimal growth and development.Pediatrics2008;122:e670–e674
A
UTOIMMUNE THYROIDITIS (AIT)is the most common inflammatory disorder of thyroid gland and manifests as either goitrous or nongoitrous forms.1The diagnosis is made on the basis of either elevated titers of antithyroid antibodies and/or fine-needle-aspiration cytology (FNAC) of the thyroid gland. This entity is being increasingly detected because of higher awareness among physicians, availability of better antibody assays, and access to FNAC. Goitrous AIT is more common in younger age groups, in whom goiter may be the only clinical manifestation.2In children with goitrous AIT, thyroid function at presentation can vary from euthyroidism to different degrees of thyroid dysfunction. The proportion of thyroid dysfunction varies widely between different studies.3–6This study was undertaken to determine the prevalence of thyroid dysfunction in young patients with goitrous AIT and rate of progression on follow-up.The natural course of autoimmunity and changes in thyroid function over time is supposed to be slow and unpredictable.1,7Studies in adults with AIT reported that progression from euthyroidism or subclinical hypothyroid-ism (SCH) to hypothyroidhypothyroid-ism is common, with chances being higher with SCH.7,8Higher thyrotropin levels at onset and thyroid antibody titers are predictive of increased chances for subsequent thyroid failure in adults.9,10Only a few studies on natural history in children with AIT are available.3,4,11Rallison et al4found that thyroid function recovered
www.pediatrics.org/cgi/doi/10.1542/ peds.2008-0493
doi:10.1542/peds.2008-0493
Key Words
autoimmune thyroiditis, natural history, thyroid function, hypothyroidism, subclinical hypothyroidism, juvenile
Abbreviations
AIT—autoimmune thyroiditis FNAC—fine-needle-aspiration cytology SCH—subclinical hypothyroidism INMAS—Institute of Nuclear Medicine and Allied Sciences
AMc—antimicrosomal antibody ATg—antithyroglobulin antibody
Accepted for publication May 12, 2008
in half of the children studied, regardless of levothyrox-ine therapy. A recently published study concluded that more than half of the children with euthyroidism re-mained so even after 4 years, whereas nearly half of those with SCH developed hypothyroidism.11
The clinical course and evolution of thyroid dysfunc-tion in young patients newly detected with goitrous AIT was studied. The study was conducted in the thyroid clinic of the Institute of Nuclear Medicine and Allied Sciences (INMAS), a tertiary-level referral center.
METHODS
Subjects and Study Protocol
The thyroid clinic of INMAS is a tertiary-level referral center, and patients are referred from all over north India. All of those presenting with goiter are subjected to a predefined set of investigations: serum thyroxine (T4), serum thyrotropin, antithyroid antibodies (antimicroso-mal [AMc] and antithyroglobulin [ATg]), and FNAC. All of the children and adolescents in the age range of 6 to 18 years presenting with diffuse goiter and diagnosed to have AIT on FNAC were consecutively recruited for the study. The study was approved by the research advisory panel of the Defense Research and Development Organi-sation under the Ministry of Defense. Informed, written consent was obtained from patients or the adolescents’ legal guardians.
All of those recruited were grouped into 3 groups based on thyroid functional status as euthyroidism (group 1), SCH (group 2), and hypothyroidism (group 3). SCH was diagnosed if thyrotropin was elevated in the presence of normal thyroxine. Overt hypothyroidism was diagnosed if thyroxine was below normal and thy-rotropin was above normal.
Follow-up
All of the patients were prospectively followed up for a minimum period of 2 years. Subjects with euthyroidism and those with SCH were monitored for thyroid function every 6 months and treated only if hypothyroidism de-veloped.
Patients with hypothyroidism (group 3) were started on levothyroxine replacement at a dose of 1.5g/kg of body weight. Serum thyrotropin estimation at 12 weeks after the initiation of therapy was used to fine tune the dose of thyroxine. Additional follow-up was performed at 6-monthly intervals. The aim of replacement therapy was to maintain serum thyrotropin in the normal range. Data on status of thyroid function and antibodies were collected at the end of study.
Laboratory Tests
Serum thyroxine and thyrotropin were estimated by radioimmunoassay and immunoradiometric assay, re-spectively. The kits were obtained from Bhabha Atomic Research Centre (Mumbai, India). The reference range of normal values for thyroxine is 4.5 to 12.5g/dL and for thyrotropin is 0.25 to 5.00IU/mL. Antithyroid an-tibodies AMc and ATg were estimated by commercially available semiquantitative hemagglutination (Serodia)
kits obtained from Fujerobio Inc (Tokyo, Japan). Test results were considered positive only if titers were⬎1: 400.
Fine-needle aspiration of thyroid was performed by an experienced cytopathologist. Aspiration was per-formed using a 23-gauge needle from 2 to 3 sites of both lobes of the thyroid. Slides were stained with May-Grunwald-Giemsa stain and observed for features of lymphocytic thyroiditis. AIT was reported if smears showed dense lymphocytic infiltration, large number of Hurthle cells in sheets, and minimal-to-moderate fibro-sis.12
RESULTS
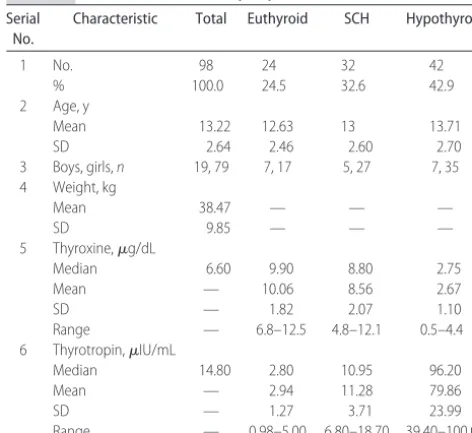
Ninety-eight subjects in the age range of 8 to 18 years were studied. Of these, 79 were girls. Characteristics of the study population and the subgroups are given in Table 1.
In 18 patients (18.4%), there was history of thyroid illness in the family: hypothyroidism in 16 and hyper-thyroidism in 2. Three patients (3.1%) had a family history of rheumatoid arthritis.
Antibodies and FNAC
The diagnosis of AIT in this study was made on the basis of FNAC. AMc titers were found to be positive in 75 subjects (76.5%), whereas ATg titer was found elevated in 54 subjects (55%).
Thyroid Function
All of the patients included into the study were grouped according to the thyroid functional status on entry. At the onset of study, 24 (24.5%) had euthyroidism, 32 subjects (32.6%) had SCH, and the remaining 42 (42.9%) had hypothyroidism. The mean⫾SD values of serum thyroxine for groups with euthyroidism, SCH,
TABLE 1 Characteristics of Study Population Serial
No.
Characteristic Total Euthyroid SCH Hypothyroid
1 No. 98 24 32 42
% 100.0 24.5 32.6 42.9
2 Age, y
Mean 13.22 12.63 13 13.71
SD 2.64 2.46 2.60 2.70
3 Boys, girls,n 19, 79 7, 17 5, 27 7, 35 4 Weight, kg
Mean 38.47 — — —
SD 9.85 — — —
5 Thyroxine,g/dL
Median 6.60 9.90 8.80 2.75
Mean — 10.06 8.56 2.67
SD — 1.82 2.07 1.10
Range — 6.8–12.5 4.8–12.1 0.5–4.4 6 Thyrotropin,IU/mL
Median 14.80 2.80 10.95 96.20
Mean — 2.94 11.28 79.86
SD — 1.27 3.71 23.99
Range — 0.98–5.00 6.80–18.70 39.40–100.00
and hypothyroidism were 10.06 ⫾ 1.82, 8.56 ⫾ 2.07, and 2.67⫾1.1g/dL, respectively. The median values of thyrotropin for the 3 groups were 2.80, 10.95, and 96.20IU/mL, respectively. There were no cases of hy-perthyroidism. All of the patients were followed up with periodic assessment of thyroid function for a minimum of 2 years.
Fifty-six patients who had either euthyroidism or SCH at onset were followed up without therapy. In group 1, of the 24 subjects who presented with euthy-roidism, 17 remained euthyroid and SCH developed in 4, whereas overt hypothyroidism developed in 3 patients at the end of follow-up.
In group 2 with SCH, of 32 subjects, 7 had euthyroid-ism and 21 continued to have SCH, whereas 4 patients developed hypothyroidism during follow-up.
Patients (group 3) who were hypothyroid were put on levothyroxine therapy, and dosage was adjusted with serum thyrotropin estimation after 12 weeks of initia-tion of therapy. All of the subjects with hypothyroid continued to require levothyroxine therapy during the period of follow-up.
Change in Antibody Profile
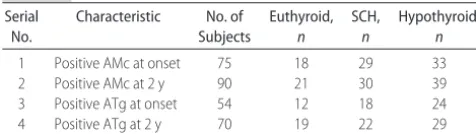
As shown in Table 2, AMc titer became positive in 15 more patients during follow-up, and ATg titer became positive in 16 patients. When the titers were analyzed, AMc increased in 30 and reduced in 60, whereas ATg increased in 20 and reduced in 67. There was no corre-lation between change in antibody titers and thyroid function.
DISCUSSION
In our study, 98 patients with goitrous AIT were evalu-ated to determine the prevalence of thyroid dysfunction and the course of autoimmunity on follow-up. When thyroid function at onset was analyzed, we found eu-thyroidism in 24%, SCH in 33%, and hypoeu-thyroidism in 43%. Although subjects with hypothyroid remained so and continued to require thyroxine replacement during the study, a small proportion of subjects in the groups with SCH or euthyroidism progressed to hypothyroid-ism.
It is well known that a firm and enlarged thyroid gland on clinical examination, along with significant titers of antithyroid antibodies, is sufficient to diagnose AIT.13 We recruited only those with diffuse goiter and AIT on FNAC to provide uniformity of the study popu-lation for follow-up. It also enabled us to see the changes in thyroid function and antibodies with time. The few
studies available in children with AIT have relied on antithyroid antibodies or sonography to diagnose AIT.
The age range and female predominance in our study are similar to several studies reported previously.3,5 Fam-ily history of thyroid illness was elicited in 18% of pa-tients in the present study. This was reported to be 27% by Sklar et al3and 33% by Desai Karandikar.14Familial aggregation of AIT is known, and antithyroid antibodies were found to be highly prevalent in first-degree rela-tives of children with AIT.1The issue of screening for AIT in young relatives of index case subjects with AIT has yet to be clarified.
Thyroid function in AIT can vary from euthyroidism to varying degrees of thyroid dysfunction, either overt or subclinical forms of hypothyroidism and hyperthyroid-ism. Although we did not find thyroid hyperfunction in any of our patients, the proportion of thyroid dysfunc-tion and euthyroidism found in this study was similar to that reported in 2 other studies that had also used lab-oratory parameters to assess thyroid function.3,5Sklar et al3found 40% to have euthyroidism, 33% to have SCH, and 27% to have hypothyroidism.
Early studies on AIT in children relied on clinical examination or indirect methods for assessment of thy-roid function.4,6 Rallison et al4found that a majority of subjects with chronic lymphocytic thyroiditis were clin-ically euthyroid (54 of 62), whereas 2 had hyperthyroid-ism and 6 had hypothyroidhyperthyroid-ism. Another study by Grun˜eiro de Papendieck et al6found only 7% to have euthyroidism, whereas 50% had hypothyroidism. Zois et al15studied 29 children with AIT and found 22 to have euthyroidism and 7 to have SCH. These children were selected from a goiter survey conducted in Greece, whereas our patients had been referred to INMAS for evaluation of goiter.
It is significant that 75% of our patients had thyroid dysfunction, which can have profound effects on growth in this age group. Acquired hypothyroidism in children and adolescents can lead to growth failure and, even if corrected, can result in suboptimal growth.16 A recent review on juvenile AIT suggested that goiter screening and thyroid function assessment in those with thyro-megaly should be made part of the routine examination in children.17
Cytological evidence of AIT formed the basis for di-agnosis and inclusion in our study. The concordance between FNAC and AMcs is very good in our study, with the latter occurring in high titers in 76% of subjects. This concordance came down to 55% in the case of ATgs. ATgs have been reported in ⬃60% of patients with diffuse goiter, hypothyroidism, or both and AMcs in 95%.18Sklar et al3found AMc titers higher than 1:100 in 29 of 30 children and ATg higher than 1:20 in 7 children. It seems that AMcs/antithyroid peroxidase antibodies alone need to be estimated in children with diffuse goiter if AIT is suspected and facilities for FNAC do not exist.
Subjects with hypothyroid (n⫽42) were given levo-thyroxine replacement to maintain thyrotropin within range. It is a generally accepted view that thyroxine replacement, which maintains a normal thyrotropin level, will normalize serum and tissue thyroid hormone TABLE 2 Evaluation of Antibodies on Follow-up
Serial No.
Characteristic No. of Subjects Euthyroid, n SCH, n Hypothyroid, n
concentration in a manner that mimics the physiologic state. These patients maintained euthyroidism on levo-thyroxine during the period of study. The findings of some studies do suggest that hypothyroidism in some cases of juvenile AIT is reversible.3,4,19 In 2 reports on goitrous thyroiditis, 1 from Japan and the other from the United States, it was seen that thyroid function re-mained normal after cessation of thyroxine therapy in at least some subjects.3,20 The presence of goiter and high thyroglobulin levels in serum may predict recovery of thy-roid function.7,21Although withdrawal of levothyroxine for a month is suggested as the method to assess whether hypothyroidism persists,7 no attempt was made to stop medication for the purpose of re-evaluation in our study, because this was not planned.
Of the 24 subjects with euthyroid, 14 continued to be so, with only 3 developing hypothyroidism on follow-up. Although it is widely held that patients with AIT progress from euthyroidism to hypothyroidism with time, recent evidence from children shows that most of them remain euthyroid but they do need periodic assess-ment of thyroid function.3,9,15
In the group with SCH, 21 of 32 children continued to have SCH and 7 reverted to euthyroidism, whereas only a small number of subjects (n ⫽ 4) developed overt hypothyroidism. In a cohort from Greece, it was ob-served that 24% of children who had SCH at onset of the study continued to be so on follow-up, whereas another 14% developed SCH later.15 In another recent study, when children with juvenile AIT and SCH were fol-lowed, from among 55 subjects, 16 reverted to normal and 16 remained so, whereas 23 developed hypothy-roidism.11
The risk of overt hypothyroidism developing in adult subjects with SCH is estimated to be 2.6% per year in the absence of anti-thyroid peroxidase antibodies and 4.3% in their presence.8,22The decision regarding treatment of SCH continues to be a matter of debate, as is evident from the difference in conclusions between 2 expert panels.23,24One expert panel reviewed currently avail-able evidence and concluded that patients with normal free thyroxine and thyrotropin ⬎10 IU/mL may be treated, whereas it advised follow-up of subjects with thyrotropin in the range of 4.5 to 10.0 IU/mL, citing insufficient evidence to support treatment.23 This was mainly because of its concern about overtreatment with levothyroxine and the resultant adverse effects on bone mineral health and heart. On the other hand, a joint statement of experts from 3 endocrine societies (Amer-ican Association of Clinical Endocrinologists, Amer(Amer-ican Thyroid Association, and the Endocrine Society) advo-cate for screening for subclinical thyroid disease, and treatment of SCH even in those with thyrotropin in the range of 4.5 to 10.0 IU/mL on the precinct that lack evidence of benefit does not necessarily mean lack of benefit.24Although both panels did not address the issue of SCH in the pediatric population, treatment of children with normal free thyroxine and thyrotropin ⬎10 IU/mL and follow-up of those with thyrotropin in the range of 4.5 to 10.0IU/mL were suggested in a recent review on juvenile AIT.17This is further compounded by
the argument of experts to revise the reference range for thyrotropin to 0.3 to 3.0IU/mL in light of data from the National Health and Nutrition Examination Survey in the United States25and that any value above this range must be considered to be early thyroid failure. However, such an approach may result in an enormous burden of diseased population, and the cost/benefit ratio needs to be found.
CONCLUSIONS
Young patients who present with goiter should be eval-uated for AIT and thyroid function. Those with euthy-roidism or SCH at the time of diagnosis need to be followed up with periodic assessment of thyroid func-tion, which will aid in early detection of thyroid dys-function to prevent adverse effects on growth and de-velopment.
REFERENCES
1. Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med.1996;335(2):99 –107
2. Brown RS. Thyroid disease in infancy, childhood and adoles-cence. In: Braverman LE, ed.Diseases of Thyroid. Totowa, NJ: Humana Press; 1997
3. Sklar CA, Qazi R, David R. Juvenile autoimmune thyroiditis. Am J Dis Child.1986;140(9):877– 880
4. Rallison ML, Dobyns BM, Keating FR, Rall JE, Tyler FH. Oc-currence and natural history of chronic lymphocytic thyroiditis in childhood.J Pediatr.1975;86(5):675– 682
5. Greenberg AH, Czernichow P, Hung W, Shelley W, Winship T, Blizzard RM. Juvenile chronic lymphocytic thyroiditis: clinical, laboratory and histological correlations.J Clin Endocrinol Metab. 1970;30(3):293–301
6. Grun˜eiro de Papendieck L, Iorcansky S, Rivarola MA, Bergada C. Variations in clinical, hormonal and serological expressions of chronic lymphocytic thyroiditis (CLT) in children and ado-lescents.Clin Endocrinol (Oxf).1982;16(1):19 –28
7. Amino N, Tada H, Hidaka Y. Chronic (Hashimoto’s) thyroiditis. In: DeGroot LJ, Jameson JL, eds.Endocrinology. 4th ed. Phila-delphia, PA: WB Saunders; 2001:1471–1480
8. Vanderpump MPJ, Tunbridge WMG, French JM, et al. The incidence of thyroid disorders in the community: a twenty year followup of the Whickham survey.Clin Endocrinol (Oxf).1995; 43:55– 68
9. Wang C, Crapo LM. The epidemiology of thyroid disease and implication for screening.Endocrinol Metab Clin N Am. 1997; 26(1):189 –218
10. Gordin A, Lamberg BA. Spontaneous hypothyroidism in symp-tomless autoimmune thyroiditis: a long-term followup study. Clin Endocrinol (Oxf).1981;15(6):537–543
11. Radetti G, Gottardi E, Bona G, Corrias A, Salardi S, Loche S. The natural history of euthyroid Hashimoto’s thyroiditis in children.J Pediatr.2006;149(6):827– 832
12. Orell SR, Philips J.The Thyroid: Fine Needle Biopsy and Cytological Diagnosis of Thyroid Lesions. Basel, Germany: Karger; 1997: 66 –72
13. Weetman AP, McGregor AM. Autoimmune thyroid disease: developments in our understanding. Endocr Rev. 1984;5(2): 309 –355
14. Desai MP, Karandikar S. Autoimmune thyroid disease in childhood: a study of children and their families.Indian Pediatr. 1999;36(7):659 – 668
deficiency in Northwestern Greece. Thyroid. 2006;16(3): 289 –293
16. Rivkees SA, Bode HH, Crawford JD. Long term growth in juvenile acquired hypothyroidism: the failure to achieve nor-mal adult stature.N Engl J Med.1988;318(10):599 – 602 17. Gopalakrishnan S, Marwaha RK. Juvenile autoimmune
thy-roiditis.J Paed Endocrinol Metab.2007;20(9):961–970 18. Amino N, Hagen SR, Yamada N, Refetoff S. Measurement of
circulating thyroid microsomal antibodies by the tanned red cell hemagglutination technique: its usefulness in the diagnosis of autoimmune thyroid diseases.Clin Endocrinol (Oxf). 1976; 5(2):115–125
19. Maenpaa J, Raatikka M, Rasanen J, Taskinen E, Wager O. Natural course of juvenile autoimmune thyroidits.J Pediatr. 1985;107(6):898 –904
20. Okamura K, Sato K, Ikenoue H, et al. Primary hypothyroidism manifested in childhood with special reference to various types of reversible hypothyroidism. Eur J Endocrinol.1994;131(2): 131–137
21. Yoshinari M, Okamura K, Tokuyama T, et al. Clinical
implica-tions of reversibility in primary goitrous hypothyroidism.BMJ. 1983;287(6394):720 –722
22. Kabadi UM. Subclinical hypothyroidism: natural course of the syndrome during a prolonged followup study.Arch Intern Med. 1993;153(8):957–961
23. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease. Scientific review and guidelines for diagnosis and manage-ment.JAMA.2004;291(2):228 –338
24. Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT. Consensus statement: subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association and the Endocrine Society. J Clin Endocrinol Metab. 2005;90(1): 581–585
DOI: 10.1542/peds.2008-0493 originally published online August 4, 2008;
2008;122;e670
Pediatrics
Ambardar, Maheshwar Sahoo and Rajan Sankar
Sripathy Gopalakrishnan, Pradeep Kumar Chugh, Mitrabasu Chhillar, Vinod Kumar
Study
Goitrous Autoimmune Thyroiditis in a Pediatric Population: A Longitudinal
Services
Updated Information &
http://pediatrics.aappublications.org/content/122/3/e670
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/122/3/e670#BIBL
This article cites 20 articles, 0 of which you can access for free at:
Subspecialty Collections
ub
http://www.aappublications.org/cgi/collection/metabolic_disorders_s
Metabolic Disorders
http://www.aappublications.org/cgi/collection/endocrinology_sub
Endocrinology
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2008-0493 originally published online August 4, 2008;
2008;122;e670
Pediatrics
Ambardar, Maheshwar Sahoo and Rajan Sankar
Sripathy Gopalakrishnan, Pradeep Kumar Chugh, Mitrabasu Chhillar, Vinod Kumar
Study
Goitrous Autoimmune Thyroiditis in a Pediatric Population: A Longitudinal
http://pediatrics.aappublications.org/content/122/3/e670
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.