CHEMISTRY
Copyright© by Houghton Mifflin Company. All rights reserved.
Chapter
Chapter
10
10
Copyright© by Houghton Mifflin Company. All rights reserved. 3
10.1-2 Intermolecular forces
Objective: Students will be able to
describe the various types of
Copyright© by Houghton Mifflin Company. All rights reserved. 4
Why are some substances solids, while
others are liquids or gases at room
Copyright© by Houghton Mifflin Company. All rights reserved. 6
solid
liquid
gas
Similar density
~ 1000
for most; solids
times
generally more less
Copyright© by Houghton Mifflin Company. All rights reserved. 7
Review of intermolecular forces
Review of intermolecular forces
1)
ion-dipole
2)
dipole – dipole (incl H bonding)
3)
dipole – induced dipole
4)
instantaneous dipole – induced
dipole
Ex:
Na
+
& H
2
O
mixture
H
2
O
H
2
O & Cl
2
mixture
I
2
Note: All of these are ~ 10 – 15 % of the strength of
bond energies.
“
London dispersion forces
”
Copyright© by Houghton Mifflin Company. All rights reserved. 8
Copyright© by Houghton Mifflin Company. All rights reserved. 9
Dipole-dipole interactions result in
higher mp and bp for similar sized
Copyright© by Houghton Mifflin Company. All rights reserved. 10
Hydrogen bonding
Copyright© by Houghton Mifflin Company. All rights reserved. 11
Hydrogen bonding
Hydrogen bonding
An attraction between a
hydrogen atom on one
Copyright© by Houghton Mifflin Company. All rights reserved. 12
… or sometimes on the same molecule,
as in DNA.
Copyright© by Houghton Mifflin Company. All rights reserved. 13
The boiling points of covalent hydrides
The boiling points of covalent hydrides
High bp due
to hydrogen
bonding
Copyright© by Houghton Mifflin Company. All rights reserved. 15
Think of e
-movement as a
swarm of bees. The
more e
-
s, the more
movement, and the
greater the
dispersion forces.
Can you explain why
F
2
and Cl
2
are gases
but Br
2
is a liquid
Copyright© by Houghton Mifflin Company. All rights reserved. 16
Figure 14.7: The heating/cooling curve for
Figure 14.7: The heating/cooling curve for
water heated or cooled at a constant rate.
water heated or cooled at a constant rate.
Copyright© by Houghton Mifflin Company. All rights reserved. 17
Figure 14.8: Both
Figure 14.8: Both
liquid water and
liquid water and
gaseous water
gaseous water
contain H
contain H
2
2
O
O
molecules.
Copyright© by Houghton Mifflin Company. All rights reserved. 18
Figure 14.9: Microscopic view of a liquid
Figure 14.9: Microscopic view of a liquid
near its surface.
Copyright© by Houghton Mifflin Company. All rights reserved. 19
Figure 14.10: Behavior of a liquid in a closed
Figure 14.10: Behavior of a liquid in a closed
container.
Copyright© by Houghton Mifflin Company. All rights reserved. 20
Figure 14.11: (a) Measuring vapor of a liquid by using a
Figure 14.11: (a) Measuring vapor of a liquid by using a
simple barometer.
simple barometer.
(b) The water vapor pushed the mercury level down.
(b) The water vapor pushed the mercury level down.
(c) Diethyl ether shows a higher vapor pressure than
(c) Diethyl ether shows a higher vapor pressure than
water.
Copyright© by Houghton Mifflin Company. All rights reserved. 21
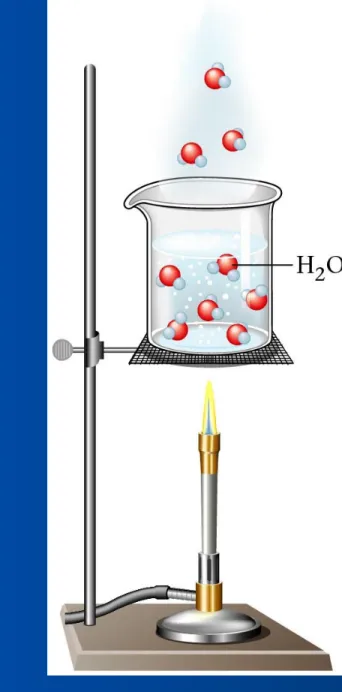
Figure 14.12: Water rapidly boiling on a stove.
Copyright© by Houghton Mifflin Company. All rights reserved. 22
Figure 14.13: Bubble expands as H
Figure 14.13: Bubble expands as H
2
2
O molecules
O molecules
enter.
Copyright© by Houghton Mifflin Company. All rights reserved. 23
Figure 14.14: The formation of the bubble is
Figure 14.14: The formation of the bubble is
opposed by atmospheric pressure.
End of lesson
10.3 – 4 Types of solids
10.3 – 4 Types of solids
Objective
Objective
: Students will be able to
: Students will be able to
recognize various types of solid crystal
recognize various types of solid crystal
structures, and carry out relevant
structures, and carry out relevant
calculations.
calculations.
Note
Note
: This topic is omitted on the current
: This topic is omitted on the current
AP exam.
AP exam.
Crystalline solids - solids with a regular particle
Crystalline solids - solids with a regular particle
arrangement.
arrangement.
Amorphous solids - solids with disordered structure.
Amorphous solids - solids with disordered structure.
Lattice - a 3-D system of points designating the positions
Lattice - a 3-D system of points designating the positions
of the particles in a crystal.
of the particles in a crystal.
Unit cell - the smallest repeating unit of a crystal lattice.
Unit cell - the smallest repeating unit of a crystal lattice.
See fig. 10.9, p. 432
See fig. 10.9, p. 432
X-ray diffraction - common way of determining
X-ray diffraction - common way of determining
crystalline structures
crystalline structures
.
.
a. metallic - delocalized electrons – the e- sea
a. metallic - delocalized electrons – the e- sea
model.
model.
b. network - atoms bond together with strong
b. network - atoms bond together with strong
covalent bonds, forming giant molecules.
covalent bonds, forming giant molecules.
c. group 8A - noble gas atoms attracted to each
c. group 8A - noble gas atoms attracted to each
other
Section 10.4 - Structure and bonding in
Section 10.4 - Structure and bonding in
metals
metals
Metallic properties such as high thermal and
Metallic properties such as high thermal and
electrical conductivity, malleability and
electrical conductivity, malleability and
ductility, are explained by non-directional
ductility, are explained by non-directional
covalent bonding.
covalent bonding.
Closest packing - a model which pictures a
Closest packing - a model which pictures a
metallic crystal as containing spherical atoms
metallic crystal as containing spherical atoms
packed together and bonded in all directions,
packed together and bonded in all directions,
to most efficiently use the available space. See
to most efficiently use the available space. See
p. 432.
A. Simple cubic
Like oranges stacked
up on top of each
other… lined up
perfectly. (Not a
closest-packing
arrangement)
B. Cubic closest packed (ccp/fcc) structure -
B. Cubic closest packed (ccp/fcc) structure -
an abc arrangement (the atoms in the first
an abc arrangement (the atoms in the first
layer do not lie directly above the atoms in
layer do not lie directly above the atoms in
the third layer) with a face-centered cubic
the third layer) with a face-centered cubic
unit cell. See p. 437.
unit cell. See p. 437.
C. Hexagonal closest packed (hcp/bcc)
C. Hexagonal closest packed (hcp/bcc)
structure - an aba arrangement (the atoms
structure - an aba arrangement (the atoms
in the first layer lie directly above the atoms
in the first layer lie directly above the atoms
in the third layer) has a hexagonal unit cell;
in the third layer) has a hexagonal unit cell;
aka body-centered cubic. See p. 437.
aka body-centered cubic. See p. 437.
3a
3a
2
2
= (4r)
= (4r)
2
2
Summary
# of atoms/unit
cell
A) simple cubic
1
B) fcc/ccp/abca
4
Sample math problem
Sodium’s density is 0.971 g/cm
3
and it
crystallizes with a body-centered cubic
unit cell.
(a) What is the radius of a sodium atom?
(b) What is the edge length of the cell?
Solution:
1) Determine mass of two atoms in a bcc cell:
2 atoms 22.99 g mol = 7.635 x 10
-23
g
mol 6.022 x 10
23
atoms
2) Determine the volume of the unit cell using V = m/D
7.635 x 10
-23
g cm
3
. = 7.863 x 10
-23
cm
3
0.971 g
3) Determine the edge length -- the answer to (b):
4) Use the Pythagorean
theorem to find the
radius.
x
2
+ x
2
+ x
2
= (4r)
2
3x
2
= 16r
2
r
2
= 3(4.284 x 10
-8
)
2
16
r = 1.855 x 10
-8
cm
The radius of the Na
atom is 185.5 pm. The
edge length is 428.4 pm.
Sample problem 2
Gold is a face centered cubic unit cell. D =
19.32 g/cm
3
. Find the radius of the gold atom
Solution:
1) Determine mass of four atoms in an fcc cell:
4 atoms 196.97 g mol = 1.308 x 10
-21
g
mol 6.022 x 10
23
atoms
2) Find the volume of the unit cell using V = m/D
1.308 x 10
-21
g cm
3
= 6.772 x 10
-23
cm
3
19.32 g
3) Find the edge length
Bonding in most metals is both strong
Bonding in most metals is both strong
and non directional. That is, although
and non directional. That is, although
it is difficult to separate metal atoms,
it is difficult to separate metal atoms,
it is relatively easy to move them,
it is relatively easy to move them,
provided the atoms stay in contact
provided the atoms stay in contact
with each other.
Molecular orbital (MO) (or band model)
Molecular orbital (MO) (or band model)
- in this model, the e-s travel around
- in this model, the e-s travel around
the metal crystal in MOs formed from
the metal crystal in MOs formed from
the valence atomic orbitals of the metal
the valence atomic orbitals of the metal
atoms. When many metal atoms
atoms. When many metal atoms
interact, the large number of resulting
interact, the large number of resulting
molecular orbitals become more closely
molecular orbitals become more closely
spaced and finally form a virtual
spaced and finally form a virtual
continuum of levels, called bands.
continuum of levels, called bands.
See fig 10.19 and 10.20
End of lesson
10.5-6 Network solids;
molecular solids
Objective: Students will be able to
Ex.
mp
H
fusion
Held
together by
metals
Al
varies
high
cations & e
-
s
nonpolar
molecular
solids
I
2
, CO
2
low
low
London
dispersion
forces
polar
molecular
solids
H
2
O
medium
medium dipole
interactions/
H bonding
network
solids
diamond,
graphite,
SiO
2
v. high
v. high
covalent
bonds
ionic solids NaCl
v. high
v. high
ionic bonds
(obviously)
amorphous
Network solids and amorphous solids.
Can you match them?
a.
silica (SiO
2
)
b.
diamond
c.
glass
d.
graphite
1.
2.
3.
Silica (SiO
2
)
Each Si is covalently bonded
to four O atoms. Net
formula = SiO
2
.
No
bonds; very different
from CO
2
silica
silicates
glass
similarities: all contain Si, O
network
solid
ionic
compounds
amorphous
SiO
2
O/Si ratio >
2:1
formed by
heating silica
Si
single-bonded to 4
O (shared)
Copyright© by Houghton Mifflin Company. All rights reserved. 46
Figure 14.15: Sodium and chloride ions.
Copyright© by Houghton Mifflin Company. All rights reserved. 47
Figure 14.18: A molecular solid.
Copyright© by Houghton Mifflin Company. All rights reserved. 48
Figure 14.19: The packing of Cl¯ and Na
Figure 14.19: The packing of Cl¯ and Na
+
+
ions in
ions in
solid sodium chloride.
Copyright© by Houghton Mifflin Company. All rights reserved. 49
Solid P
Alloy
Alloy
- a mixture of
- a mixture of
elements with metallic
elements with metallic
properties.
properties.
1. Substitutional alloy -
1. Substitutional alloy -
some of the host metal
some of the host metal
atoms are replaced by
atoms are replaced by
other metal atoms of similar
other metal atoms of similar
size.
size.
2. Interstitial alloy - formed
2. Interstitial alloy - formed
when some of the
when some of the
interstices (holes) in the
interstices (holes) in the
closest packed metal
closest packed metal
structure are occupied by
structure are occupied by
small atoms.
Copyright© by Houghton Mifflin Company. All rights reserved. 51
Brass… which type of alloy is this?
Copyright© by Houghton Mifflin Company. All rights reserved. 52
Steel… Which type of alloy is this?
Copyright © Cengage Learning. All rights reserved 53
As intermolecular forces
increase, what
happens to each of the following? Why?
boiling point
viscosity
surface tension
enthalpy of fusion
freezing point
vapor pressure
heat of vaporization
End of section
10.8 Vapor pressure and
changes of state
Objective: Students will be able to
describe vapor pressure and carry
Vaporization
Vaporization – the process of a liquid
changing to a gas
H
vap
– heat of vaporization - the heat
required to vaporize 1 mole of a liquid at
1 atm pressure
Water has a very high
H
vap
(40.7 kJ/mol)
due to strong hydrogen bonding.
Vapor pressure
Behavior of a liquid in a closed container
Behavior of a liquid in a closed container
Different liquids have
different vapor pressure.
Weak intermolecular attractions
higher v.p.
more volatile (evaporates
readily)
Vapor pressure
Notice as T
v.p.
.
The mathematical relationship is given by
the Clausius-Clapeyron equation:
l
n(P
vap
) = -
H
vap
1 + C
R T
l
n(P
vap
) = -
H
vap
1 + C
R T
This means a plot of
l
n(P
vap
) versus 1/T
(reciprocal of Kelvin temp) would give a
straight line with slope –
H
vap
/R.
8
7
6
5
4
3
2
1
0
l
n
P
slope =
y = 0 - 8.00
x (4.03 x 10
-3
K
-1
) - (2.75 x 10
-3
K
-1
)
-8.00 = -6.25 x 10
3
K
1.28 x 10
-3
K
-1
slope = -
H
R
H = -slope · R = (+6.25 x 10
3
K) · (8.3145
Clausius – Clapeyron Equation
Clausius – Clapeyron Equation
P
vap
= vapor pressure
Δ
H
vap
= enthalpy of vaporization
R
= 8.3145 J/K·mol
End of lesson
End of lesson
See homework on board.
10.9 Phase diagrams
Objective: Students will be able to
identify key points on a phase
diagram and use a phase diagram to
describe important characteristics of a
substance.
Note: This topic is omitted on the
Copyright © Cengage Learning. All rights reserved 69
Heating Curve for Water
Copyright © Cengage Learning. All rights reserved 70
phase diagram: a convenient way of representing
the phases of a substance as a function of
temperature and pressure. It shows:
triple point (s, l, g all exist at equilibrium)
critical point (T and P above which gas and liquid
phases are indistinguishable)
Copyright © Cengage Learning. All rights reserved 71
Phase
Phase
diagram
diagram
L
G
S
A typical phase
diagram. The
solid green line
applies to most
substances; the
dotted green line
gives the
anomalous
Copyright © Cengage Learning. All rights reserved 72
Phase
Phase
diagram for
diagram for
water
Copyright © Cengage Learning. All rights reserved 73
Phase diagram for water
Phase diagram for water
Normal
melting
point
Normal
boiling
point
Solid-liquid eq’m line
Liquid-gas eq’m line
Copyright © Cengage Learning. All rights reserved 74
Phase diagram for water
Phase diagram for water
Notice:
S-L eq’m line has a negative
slope.
This means ice is less dense
than water.
When pressure is put on ice,
it melts.
AP Chem annual skating
Copyright © Cengage Learning. All rights reserved 75
Copyright © Cengage Learning. All rights reserved 76
Phase
Phase
diagram for
diagram for
carbon
carbon
dioxide
Copyright © Cengage Learning. All rights reserved 77
Phase
Phase
diagram for
diagram for
carbon
carbon
dioxide
dioxide
Notice:
S-L eq’m line has a positive slope.
This means dry ice is more dense than liquid
CO
2
.
End of lesson
See homework on board.
Extra stuff from the publishers
…
Chapter 10
Chapter 10
Copyright © Cengage Learning. All rights reserved 81
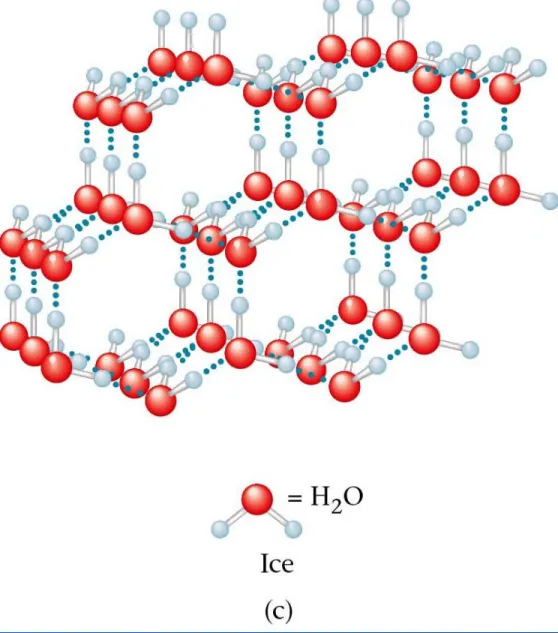
Hydrogen Bonding in Water
Hydrogen Bonding in Water
Blue dotted lines are the
Copyright © Cengage Learning. All rights reserved 82
Concept Check
Concept Check
Which are stronger, intramolecular bonds or
intermolecular forces?
Copyright © Cengage Learning. All rights reserved 83
Phase Changes
Phase Changes
When a substance changes from solid to liquid to
gas, the molecules remain intact.
The changes in state are due to changes in the forces
Copyright © Cengage Learning. All rights reserved 84
Schematic Representations of the Three States of Matter
Copyright © Cengage Learning. All rights reserved 85
Phase Changes
Phase Changes
Solid to Liquid
As energy is added, the motions of the molecules
increase, and they eventually achieve the greater
movement and disorder characteristic of a liquid.
Liquid to Gas
As more energy is added, the gaseous state is
Copyright © Cengage Learning. All rights reserved 86
Densities of the Three States of Water
Copyright © Cengage Learning. All rights reserved 87
Dipole-Dipole Forces
Dipole-Dipole Forces
Dipole moment – molecules with polar bonds often
behave in an electric field as if they had a center of
positive charge and a center of negative charge.
Molecules with dipole moments can attract each other
electrostatically. They line up so that the positive and
negative ends are close to each other.
Copyright © Cengage Learning. All rights reserved 88
Dipole-Dipole Forces
Copyright © Cengage Learning. All rights reserved 89
Hydrogen Bonding
Copyright © Cengage Learning. All rights reserved 90
Hydrogen Bonding
Hydrogen Bonding
Strong dipole-dipole forces.
Hydrogen is bound to a highly electronegative atom –
Copyright © Cengage Learning. All rights reserved 91
London Dispersion Forces
Copyright © Cengage Learning. All rights reserved 92
London Dispersion Forces
London Dispersion Forces
Instantaneous dipole that occurs accidentally in a
given atom induces a similar dipole in a neighboring
atom.
Significant in large atoms/molecules.
Copyright © Cengage Learning. All rights reserved 93
Melting and Boiling Points
Melting and Boiling Points
In general, the stronger the intermolecular forces, the
Copyright © Cengage Learning. All rights reserved 94
The Boiling Points of the Covalent Hydrides of the Elements in Groups
The Boiling Points of the Covalent Hydrides of the Elements in Groups
4A, 5A, 6A, and 7A
Copyright © Cengage Learning. All rights reserved 95
Concept Check
Concept Check
Which molecule is capable of forming stronger
intermolecular forces?
N
2
H
2
O
Copyright © Cengage Learning. All rights reserved 96
Concept Check
Concept Check
Draw two Lewis structures for the formula C
2
H
6
O
and
compare
the boiling points of the two molecules.
C
H
H
C
H
H
H
O H
C
Copyright © Cengage Learning. All rights reserved 97
Concept Check
Concept Check
Which gas would behave
more ideally
at the same
conditions of P and T?
CO or
N
2
Copyright © Cengage Learning. All rights reserved 98
Liquids
Liquids
Low compressibility, lack of rigidity, and high
density compared with gases.
Surface tension – resistance of a liquid to an increase
in its surface area:
Liquids with large intermolecular forces tend to
Copyright © Cengage Learning. All rights reserved 99
Liquids
Liquids
Capillary action – spontaneous rising of a liquid in a
narrow tube:
Cohesive forces – intermolecular forces among
the molecules of the liquid.
Adhesive forces – forces between the liquid
Copyright © Cengage Learning. All rights reserved 100
Convex Meniscus Formed by Nonpolar Liquid Mercury
Convex Meniscus Formed by Nonpolar Liquid Mercury
Which force dominates alongside the glass tube –
cohesive or adhesive forces?
Copyright © Cengage Learning. All rights reserved 101
Concave Meniscus Formed by Polar Water
Concave Meniscus Formed by Polar Water
Which force dominates alongside the glass tube –
cohesive or adhesive forces?
Copyright © Cengage Learning. All rights reserved 102
Liquids
Liquids
Viscosity – measure of a liquid
’
s resistance to flow:
Liquids with large intermolecular forces or
Copyright © Cengage Learning. All rights reserved 103
Solids
Solids
Amorphous Solids:
Disorder in the structures
Glass
Crystalline Solids:
Copyright © Cengage Learning. All rights reserved 104
Three Cubic Unit Cells
Three Cubic Unit Cells
and the Corresponding Lattices
Copyright © Cengage Learning. All rights reserved 105
Bragg Equation
Bragg Equation
Used to determine the interatomic spacings.
n = integer
= wavelength of the X rays
d = distance between the atoms
= angle of incidence and reflection
= 2 sin
n
d
Copyright © Cengage Learning. All rights reserved 106
Bragg Equation
Bragg Equation
= 2 sin
Copyright © Cengage Learning. All rights reserved 107
Types of Crystalline Solids
Types of Crystalline Solids
Ionic Solids – ions at the points of the lattice that
describes the structure of the solid.
Molecular Solids – discrete covalently bonded
molecules at each of its lattice points.
Atomic Solids – atoms at the lattice points that
Copyright © Cengage Learning. All rights reserved 108
Examples of Three Types of Crystalline Solids
Copyright © Cengage Learning. All rights reserved 109
Classification of Solids
Copyright © Cengage Learning. All rights reserved 110
Closest Packing Model
Closest Packing Model
Closest Packing:
Assumes that metal atoms are uniform, hard
spheres.
Copyright © Cengage Learning. All rights reserved 111
The Closest Packing Arrangement of Uniform Spheres
The Closest Packing Arrangement of Uniform Spheres
aba
packing – the 2
nd
layer is like the 1
st
but it is displaced so
that each sphere in the 2
nd
layer occupies a dimple in the 1
st
layer.
Copyright © Cengage Learning. All rights reserved 112
The Closest Packing Arrangement of Uniform Spheres
The Closest Packing Arrangement of Uniform Spheres
abc
packing – the spheres in the 3
rd
layer occupy dimples in the
2
nd
layer so that no spheres in the 3
rd
layer lie above any in the
1
st
layer.
Copyright © Cengage Learning. All rights reserved 113
Hexagonal Closest Packing
Copyright © Cengage Learning. All rights reserved 114
Cubic Closest Packing
Copyright © Cengage Learning. All rights reserved 115
The Indicated Sphere Has 12 Nearest Neighbors
The Indicated Sphere Has 12 Nearest Neighbors
Each sphere in both
Copyright © Cengage Learning. All rights reserved 116
The Net Number of Spheres in a Face-Centered Cubic Unit Cell
Copyright © Cengage Learning. All rights reserved 117
Concept Check
Concept Check
Determine the number of metal atoms in a unit cell if the
packing is:
a)
Simple cubic
b)
Cubic closest packing
Copyright © Cengage Learning. All rights reserved 118
Concept Check
Concept Check
A metal crystallizes in a face-centered cubic
structure.
Determine the relationship between the radius of
the metal atom and the length of an edge of the
unit cell.
Copyright © Cengage Learning. All rights reserved 119
Concept Check
Concept Check
Silver metal crystallizes in a
cubic closest packed
structure
. The face centered cubic unit cell edge is
409 pm.
Calculate the
density
of the silver metal.
Copyright © Cengage Learning. All rights reserved 120
Bonding Models for Metals
Bonding Models for Metals
Electron Sea Model
Copyright © Cengage Learning. All rights reserved 121
The Electron Sea Model
The Electron Sea Model
A regular array of cations in a
“
sea
”
of mobile
Copyright © Cengage Learning. All rights reserved 122
Molecular Orbital Energy Levels Produced When Various Numbers of
Molecular Orbital Energy Levels Produced When Various Numbers of
Atomic Orbitals Interact
Copyright © Cengage Learning. All rights reserved 123
The Band Model for Magnesium
The Band Model for Magnesium
Copyright © Cengage Learning. All rights reserved 124
Metal Alloys
Metal Alloys
Substitutional Alloy – some of the host metal atoms
are replaced by other metal atoms of similar size.
Interstitial Alloy – some of the holes in the closest
Copyright © Cengage Learning. All rights reserved 125
Two Types of Alloys
Two Types of Alloys
Brass is a
substitutional
alloy.
Steel is an
Copyright © Cengage Learning. All rights reserved 126
The Structures of Diamond and Graphite
Copyright © Cengage Learning. All rights reserved 127
Partial Representation of the Molecular Orbital Energies in
Partial Representation of the Molecular Orbital Energies in
a) Diamond b) a Typical Metal
Copyright © Cengage Learning. All rights reserved 128
The
Copyright © Cengage Learning. All rights reserved 129
Ceramics
Ceramics
Typically made from clays (which contain silicates)
and hardened by firing at high temperatures.
Nonmetallic materials that are strong, brittle, and
Copyright © Cengage Learning. All rights reserved 130
Semiconductors
Semiconductors
n-type semiconductor – substance whose
conductivity is increased by doping it with atoms
having more valence electrons than the atoms in the
host crystal.
p-type semiconductor – substance whose
conductivity is increased by doping it with atoms
Copyright © Cengage Learning. All rights reserved 131
Energy Level Diagrams for
Energy Level Diagrams for
Copyright © Cengage Learning. All rights reserved 132
Silicon Crystal Doped with
Silicon Crystal Doped with
(a) Arsenic and
(a) Arsenic and
(b) Boron
Copyright © Cengage Learning. All rights reserved 133
Three Types of Holes in Closest Packed Structures
Three Types of Holes in Closest Packed Structures
1)
Trigonal holes are formed by three spheres in the
Copyright © Cengage Learning. All rights reserved 134
Three Types of Holes in Closest Packed Structures
Three Types of Holes in Closest Packed Structures
2)
Tetrahedral holes are formed when a sphere sits in
Copyright © Cengage Learning. All rights reserved 135
Three Types of Holes in Closest Packed Structures
Three Types of Holes in Closest Packed Structures
3)
Octahedral holes are formed between two sets of
Copyright © Cengage Learning. All rights reserved 136
For spheres of a given diameter, the holes increase in
size in the order:
Copyright © Cengage Learning. All rights reserved 137
Types and Properties of Solids
Copyright © Cengage Learning. All rights reserved 138
The Rates of Condensation and Evaporation
Copyright © Cengage Learning. All rights reserved 139
Vapor Pressure
Vapor Pressure
Pressure of the vapor present at equilibrium.
The system is at equilibrium when no net change
Copyright © Cengage Learning. All rights reserved 140
Concept Check
Concept Check
What is the vapor pressure of water at 100°C? How
do you know?
Copyright © Cengage Learning. All rights reserved 141
Vapor Pressure
Copyright © Cengage Learning. All rights reserved 142
Vapor Pressure
Vapor Pressure
Liquids in which the intermolecular forces are strong
have relatively low vapor pressures.
Vapor pressure increases significantly with
Copyright © Cengage Learning. All rights reserved 143
Vapor Pressure
Copyright © Cengage Learning. All rights reserved 144
Concept Check
Concept Check
The vapor pressure of water at 25°C is 23.8 torr, and
the heat of vaporization of water at 25°C is 43.9 kJ/
mol. Calculate the vapor pressure of water at 65°C.
Copyright © Cengage Learning. All rights reserved 145
Concept Check
Concept Check
Which would you predict should be
larger
for a
given substance:
H
vap
or
H
fus
?
Copyright © Cengage Learning. All rights reserved 146
Concept Check
Concept Check
As intermolecular forces
increase
, what happens to each
of the following? Why?
Boiling point
Viscosity
Surface tension
Enthalpy of fusion
Freezing point
Vapor pressure