Factors Associated With Rotavirus
Vaccine Coverage
Negar Aliabadi, MD, MS,aMary E. Wikswo, MPH,aJacqueline E. Tate, PhD,aMargaret M. Cortese, MD,a
Peter G. Szilagyi, MD, MPH,b,cMary Allen Staat, MD, MPH,dGeoffrey A. Weinberg, MD,bNatasha B. Halasa, MD, MPH,e Julie A. Boom, MD,f,gRangaraj Selvarangan, PhD,hJanet A. Englund, MD,iParvin H. Azimi, MD,jEileen J. Klein, MD, MPH,i Mary E. Moffatt, MD,hChristopher J. Harrison, MD,hLeila C. Sahni, PhD, MPH,fLaura S. Stewart, PhD,e
David I. Bernstein, MD, MA,dUmesh D. Parashar, MBBS, MPH,aDaniel C. Payne, PhD, MSPHa
abstract
BACKGROUND:Rotavirus vaccines (RVVs) were included in the US immunization program in 2006and are coadministered with the diphtheria-tetanus-acellular pertussis (DTaP) vaccine, yet their coverage lags behind DTaP. We assessed timing, initiation, and completion of the RVV series among children enrolled in active gastroenteritis surveillance at 7 US medical
institutions during 2014–2016.
METHODS:We compared coverage and timing of each vaccine series and analyzed characteristics
associated with RVV initiation and completion. We report odds ratios (ORs) and 95%
confidence intervals (CIs) from multivariable logistic regression models.
RESULTS:We enrolled 10 603 children. In 2015,$1 dose coverage was 91% for RVV and 97%
for DTaP. Seven percent of children received theirfirst DTaP vaccine at age$15 weeks versus
4% for RVV (P#.001). Recent birth years (2013–2016) were associated with higher odds of
RVV initiation (OR = 5.72; 95% CI 4.43–7.39), whereas preterm birth (OR = 0.32; 95% CI
0.24–0.41), older age at DTaP initiation (OR 0.85; 95% CI 0.80–0.91), income between
$50 000 and $100 000 (OR = 0.56; 95% CI 0.40–0.78), and higher maternal education
(OR = 0.52; 95% CI 0.36–0.74) were associated with lower odds. Once RVV was initiated,
recent birth years (2013–2016; OR = 1.57 [95% CI 1.32–1.88]) and higher maternal education
(OR = 1.31; 95% CI 1.07–1.60) were associated with higher odds of RVV completion, whereas
preterm birth (OR = 0.76; 95% CI 0.62–0.94), African American race (OR = 0.82; 95% CI
0.70–0.97) and public or no insurance (OR = 0.75; 95% CI 0.60–0.93) were associated with
lower odds. Regional differences existed.
CONCLUSIONS:RVV coverage remains lower than that for the DTaP vaccine. Timely DTaP administration may help improve RVV coverage.
WHAT’S KNOWN ON THIS SUBJECT:Rotavirus vaccine coverage in US children lags behind diphtheria-tetanus-acellular pertussis vaccine. The Advisory Committee on Immunization Practices recommended that age restrictions for the rotavirus vaccine likely play a role; other factors associated with low uptake have not fully been described.
WHAT THIS STUDY ADDS:We identify preterm birth and older age at diphtheria-tetanus-acellular pertussis vaccine initiation as factors leading to missed opportunities for rotavirus vaccination (in addition to region-specific socioeconomic factors). We identify interventions to improve coverage and special populations for further study.
To cite:Aliabadi N, Wikswo ME, Tate JE, et al. Factors
Associated With Rotavirus Vaccine Coverage.Pediatrics.
2019;143(2):e20181824
aDivision of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease
Control and Prevention, Atlanta, Georgia;bSchool of Medicine and Dentistry, University of Rochester, Rochester,
New York;cUniversity of California, Los Angeles, Los Angeles, California;dCincinnati Children’s Hospital Medical
Center, Cincinnati, Ohio;eVanderbilt University Medical Center, Nashville, Tennessee;fTexas Children’s Hospital,
Houston, Texas;gBaylor College of Medicine, Houston, Texas;hChildren’s Mercy Hospital, Kansas City, Missouri;
iSeattle Children’s Hospital, Seattle, Washington; andjUniversity of California, San Francisco Benioff Children’s
Hospital Oakland, Oakland, California
Drs Aliabadi, Payne, Parashar, Tate, Cortese, and Szilagyi and Ms Wikswo contributed to the study design, analysis, and interpretation and the critical review and feedback of the manuscript; Ms Sahni and Drs Staat, Weinberg, Halasa, Boom, Selvarangan, Englund, Azimi, Klein, Moffatt, Harrison, Stewart, and Bernstein provided critical review and feedback for the manuscript; and all authors
approved thefinal manuscript as submitted and agree to be accountable for all aspects of
The introduction of rotavirus vaccines (RVVs) in the United States in 2006 heralded dramatic declines in rotavirus disease burden.1–3Two RVVs are licensed by the US Food and Drug Administration and recommended by the Advisory Committee on Immunization Practices (ACIP) for universal infant immunization.4Rotarix (RV1; GlaxoSmithKline Biologicals, Rixensart, Belgium) is a 2-dose monovalent RVV recommended by the ACIP to be given at 2 and 4 months of age, and RotaTeq (RV5; Merck and Company, Kenilworth, NJ) is a 3-dose pentavalent RVV
recommended to be given at 2, 4, and 6 months of age. RVVs are coadministered with other vaccines, including the diphtheria-tetanus-acellular pertussis (DTaP) vaccine.5 ACIP recommendations for RVV
include maximum ages for thefirst
dose (14 weeks, 6 days) and last dose
(8 months, 0 days)6with no catchup;
these restrictions are based on the absence of safety and efficacy data on RVV given outside these ages and do not exist for the DTaP vaccine.
RVV coverage in US children lags behind that for the DTaP vaccine. Among children aged 19 to 35 months, the National Immunization Survey (NIS) has consistently reported at least a 21 percentage point lower RVV coverage compared with that of the DTaP vaccine since 2009.7–12In 2015, the estimated 3-dose DTaP vaccine coverage was 95% (95% confidence interval [CI]
94%–96%), whereas full-series RVV
coverage (2-dose RV1 or 3-dose RV5)
was 73% (95% CI 72%–75%).12
Two other studies, from state immunization registries
(Immunization Information System [IIS] sentinel sites) and a large commercial data set, also revealed lower RVV coverage compared with that of the DTaP vaccine. The former revealed a 6 to 8 percentage point discrepancy between the DTaP
vaccine and RVV for$1 dose
coverage among children 5 months of age during 2012–2013,13whereas the latter revealed that full-course RVV coverage among children 13 months of age born in 2011–2012 trailed 3-dose DTaP vaccine coverage by 10 percentage points.14Reasons for these differences are not clear, but the child’s age at vaccination, birth year, ethnicity, and race; provider type; and urban versus rural residence have been implicated.14–17
To better understand why RVV coverage continues to be lower than that of the DTaP vaccine despite the recommendation to administer both vaccines at the same age-defined health care visits, we analyzed data from a large active acute gastroenteritis (AGE) surveillance system at 7 US medical institutions with large catchment areas. Our objectives were the following: (1) to determine the proportion of children who initiated RVV and the DTaP vaccine, (2) to examine timeliness of RVV and DTaP vaccine
administration, (3) to determine risk factors for missed opportunities to initiate RVV, and (4) to examine factors associated with RVV series completion.
METHODS
Patients
We analyzed data for children enrolled in the New Vaccine
Surveillance Network (NVSN) during
December 2014–June 2016. The
NVSN is a previously described active prospective AGE surveillance
network18in 7 sentinel sites: Nashville, Rochester, Cincinnati, Seattle, Houston, Kansas City, and Oakland. We included children born after January 1, 2007, because RVV became available in the United States
during 2006. Children with AGE ($3
episodes of diarrhea and 24 hours
and/or$1 episode of vomiting) were
enrolled and tested for rotavirus by using Rotaclone enzyme immunoassay; children who were
immunocompromised were excluded. In 6 sites, children aged 14 days to 10 years with AGE were enrolled from inpatient and emergency wards. In Nashville, outpatients with AGE were also enrolled. Healthy controls in this age range with no AGE symptoms for the previous 14 days were enrolled at well-child visits from all sites. Epidemiological and clinical data were collected from structured caregiver interviews, medical chart reviews, and verified rotavirus and DTaP immunization data from immunization registries and provider records. Approval for the study was obtained from the institutional review board at each site and from the Centers for Disease Control and Prevention.
Analysis
We examined different subsets of enrolled patients with AGE and healthy controls for each objective.
To determine coverage with$1 dose
of RVV or the DTaP vaccine, using previously described NVSN methods, we examined data from children aged 6 to 35 months with rotavirus-negative AGE (from inpatient and/or emergency department setting) during January 2015–June 2015.
All remaining analysis groups combined children with AGE (positive or rotavirus-negative) and healthy controls. Timeliness of DTaP vaccine and RVV receipt was assessed for those with verified immunization records. Among all children who received 1, 2, or 3 RVV doses, the cumulative proportion that received each dose was calculated by age; similar
calculations were made for thefirst 3
DTaP vaccine doses. The cumulative proportion of children vaccinated
with specific dose numbers of RVV
was compared with that of the DTaP vaccine. To determine if age
maximum age for thefirst RVV dose) was compared with the proportion initiating the DTaP vaccine at$15 weeks of age. Similar analyses were completed for the last dose of RVV and the third dose of the DTaP vaccine at$8 months of age.
To study missed opportunities for RVV initiation (defined as receipt of $1 RVV dose), we restricted the study population to those aged
$15 weeks at enrollment who had
received thefirst DTaP vaccine dose
within the recommended ACIP schedule for thefirst RVV dose (between 6 and 14 weeks, 6 days of age). This window reflects the recommended ACIP schedule shared for both the DTaP vaccine and RVV,
and by enrolling those aged$15
weeks, we ensured that children had
an opportunity to receive afirst RVV
dose. The outcome was RVV initiation. Covariates included birth year, preterm birth, age at DTaP vaccine initiation, race, ethnicity, household income, insurance status, mother’s age, and mother’s
educational status. Given that the Food and Drug Administration temporarily recommended
suspension of monovalent RVV from
March 22, 2010, to May 16, 2010,19
we performed a sensitivity analysis to determine if exclusion of infants born
during January 2010–June 2010
alteredfindings.
To study factors associated with RVV series completion, we restricted the study population to children enrolled
at age$8 months who had initiated
RVV. This ensured that children had the opportunity tofinish either the 2- or 3-dose RVV series by the
ACIP-recommended time of 8 months. The outcome was RVV completion, with receipt of either 2-dose monovalent RVV or 3 doses of either RVV series classified as complete. A partial series (1 dose of either RVV or 2 doses of pentavalent RVV or 2 doses of mixed monovalent and pentavalent RVV) was categorized as an incomplete RVV series.
Descriptive data are reported by using proportions andx2testing for categorical variables and medians and nonparametric Wilcoxon tests for continuous variables. An
unconditional logistic regression was used to compare potential factors associated with RVV receipt, and odds ratios (ORs) with 95% CIs are reported. Statistically significant characteristics from bivariate analyses were included in the FIGURE 1
multivariable model by using backward elimination to keep significant variables in thefinal model. Similar methods were used for the RVV completion analysis. Both analyses were stratified by site, and the same methods were used to obtain the most parsimonious multivariable models. Models yielding unstable results are not shown (a= .05).
RESULTS
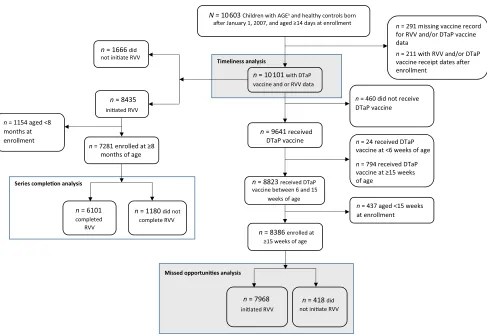
A total of 10 603 children born after January 1, 2007, were enrolled during
December 2014–June 2016. Different
subsets of this population were analyzed for timeliness and missed opportunities for RVV initiation and completion (Fig 1). Of the enrolled children, 10 101 had verified DTaP vaccine and RVV information and were included in the timeliness analysis. Of these, 8386 (83%) received the DTaP vaccine within age
6 to 15 weeks and were$15 weeks
of age at enrollment. And of these DTaP vaccine recipients, 7968 (95%) had also initiated RVV, whereas 418 (5%) had not; these children were included in the missed opportunities for RVV initiation analysis. Among the 10 101 enrollees with vaccine data available, 7281 (72%) initiated RVV
and were aged$8 months at
enrollment. Of these, 6101 (84%) completed the RVV series, whereas 1180 (16%) did not; these children were included in the missed opportunities for RVV completion analysis.
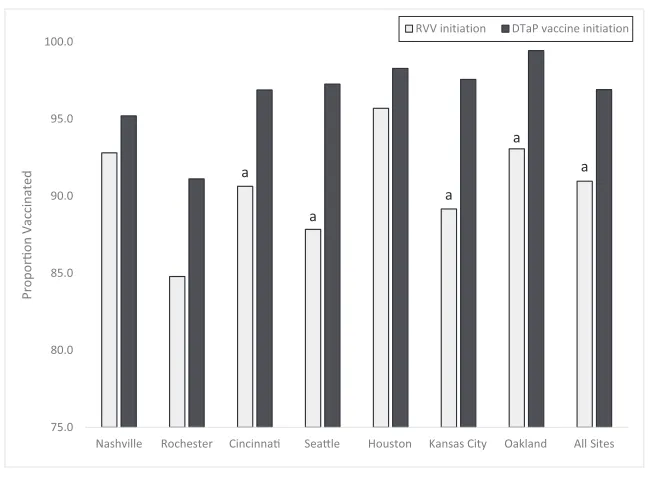
Coverage With‡1 Dose of RVV Versus DTaP Vaccine
In 2015, 91% of enrollees in the coverage cohort who were rotavirus negative initiated RVV, and 97% initiated DTaP vaccine (Fig 2). One-dose RVV coverage was lower than that of DTaP vaccine at all sites, with significant differences in Cincinnati, Seattle, Kansas City, and Oakland.
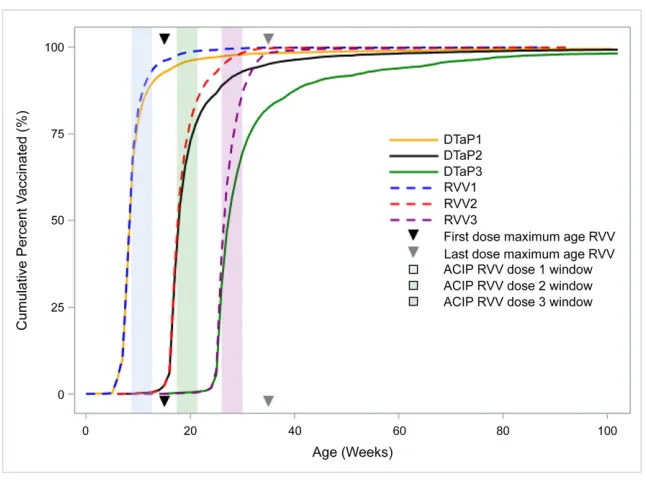
Timing of Vaccine Initiation
Of the 10 101 children with vaccine data available analyzed, 1666 never initiated RVV, and 460 never initiated the DTaP vaccine, whereas 439 did not initiate either vaccine. Among children who initiated either vaccine, 3.9% (341 of 8798) received the
first RVV dose at age$15 weeks,
compared with 7.2% (695/9641) for the DTaP vaccine (P#.001; Fig 3). The proportions that received the
doses at age$8 months was 1.5%
(65 of 4454) for last-dose RVV versus 16.2% (1308 of 8076) for third-dose DTaP vaccine (P#.001).
Missed Opportunities for RVV Initiation
Unconditional multivariable logistic regression models revealed that
children born in 2013–2016 had
5.72 times (95% CI 4.43–7.39) the odds of RVV initiation versus those
born in 2007–2009 (Table 1). Additionally, for each 1-week increase in infant age at DTaP vaccine initiation, the child’s odds of initiating RVV dropped by 15% each week (OR = 0.85; 95% CI 0.80–0.91). Other factors associated with lower odds of receiving RVV included preterm birth (OR = 0.32; 95% CI 0.24–0.41), household income
($50 001–$100 000: OR = 0.56 [95%
CI 0.40–0.78]; $100 000–$150 000: OR = 0.68 [95% CI 0.47–0.99]), and higher maternal education (OR = 0.52; 95% CI 0.36–0.74). The exclusion of 288 children born during
January 2010–June 2010 did not
appreciably change estimates (data not shown).
When stratified by site, children born in 2013–2016 had 4.18 to 12.95 higher odds of initiating RVV compared with those born in 2007–2009 (Table 2). African FIGURE 2
American infants had higher odds of receiving RVV than white infants in Cincinnati (OR = 1.97; 95% CI 1.05–3.71) and Oakland (OR = 4.20; 95% CI 1.58–11.17). Hispanic infants had twice the odds of initiating RVV versus non-Hispanic infants in Seattle
(OR = 2.23; 95% CI 1.19–4.17) and
Houston (OR = 2.45; 95% CI
1.32–4.58) but less than half the odds of non-Hispanic infants in Kansas City (OR = 0.45; 95% CI 0.26–0.78). Increasing age at DTaP vaccine administration was associated with 15% to 25% lower odds of initiating RVV in Cincinnati, Houston, and Kansas City. Mothers with higher education in Oakland had less than half the odds of RVV initiation versus those with no formal degree
(OR = 0.40; 95% CI 0.17–0.94).
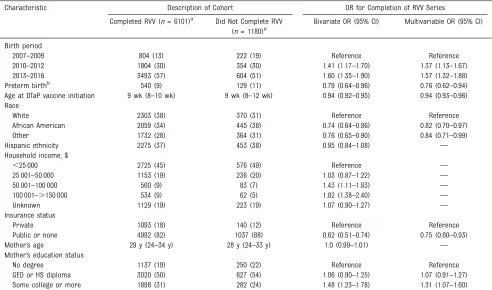
Factors Associated With RVV Completion
Among the 1180 children who did not complete the RVV series, 173 (19%) had initiated the RVV series when
aged$15 weeks. Factors associated
with higher odds of RVV completion included recent birth years (OR = 1.57 [95% CI 1.32–1.88]) and higher maternal education (OR = 1.31 [95% CI 1.07–1.60]; Table 3). Conversely, preterm birth (OR = 0.76; 95% CI 0.62–0.94), greater age at DTaP vaccine initiation (OR = 0.94; 95% CI 0.93–0.96), African American race (OR = 0.82; 95% CI 0.70–0.97), and public or no insurance (OR = 0.75;
95% CI 0.60–0.93) were associated
with lower odds of RVV completion. When stratified by site, notable differences included that Hispanic children had more than half the odds of RVV completion versus non-Hispanic children in Rochester (OR = 0.53; 95% CI 0.29–0.97), whereas African American infants in Houston had less than half the odds of RVV completion versus white infants (OR = 0.44 [95% CI 0.26–0.74]; Table 4). Mothers with higher education in Oakland had higher odds of RVV series completion versus those without high school degrees (OR = 2.06; 95% CI 1.31–3.25).
DISCUSSION
Among children enrolled in AGE surveillance at 7 large US pediatric medical institutions, RVV initiation lagged behind DTaP vaccine initiation by 6 percentage points nearly a decade after RVV’s approval for routine infant vaccination4in concordance with the 4% to 10% lag reported by IIS sites and in insurance claims studies.13,14 Concerns previously reported to contribute to lagging RVV coverage have included vaccine safety concerns related to intussusception, which resulted in the development of age restrictions for RVV administration4; concerns with vaccinating infants hospitalized in the NICU with live-attenuated virus vaccines, including RVV; detection of porcine circovirus genetic material in RVV; and
RVV-derived reassortant strains.20Our findings reveal that RVV age restrictions and lower NICU RVV vaccination practices are factors contributing to the lower odds of uptake for RVV compared with the DTaP vaccine.
Given the overlapping recommended immunization schedules for RVV and the DTaP vaccine, children presenting to a health care provider to initiate DTaP vaccination within 6 to 14 weeks, 6 days of age theoretically have the same opportunity to initiate RVV, barring contraindications or vaccine unavailability.15,17However, children outside this age window should not initiate RVV per ACIP recommendations, and we found 15% lower odds of RVV initiation with each week of increasing age at DTaP vaccine initiation. We also found that
∼4% and 2% of children received
theirfirst andfinal RVV doses after
the maximum ACIP-recommended ages, respectively, and that this occurred less often than receiving the DTaP vaccine outside these ages (7% and 16%, respectively), highlighting the rigidity of RVV recommendations. The proportion of children receiving
thefirst RVV dose outside the
recommended age is similar to that FIGURE 3
reported by the Vaccine Safety Datalink (1.4%) and the Centers for Disease Control and Prevention IIS
(5.0%)21in studies conducted soon
after RVV introduction. Insurance claims data have revealed that coverage for RVV, the DTaP vaccine, and the pneumococcal conjugate vaccine, which are coadministered, diverges at 8 months.14In that study of infants born between 2009 and 2012, coverage for these 3 vaccines
was similar in thefirst 7 months of
life; RVV coverage increased only from 69% to 73% between 7 and 13 months, whereas DTaP vaccine coverage increased from 73% to 83%, and pneumococcal conjugate vaccine coverage increased from 69% to 84%. In Australia, although RVV age restrictions may have improved the timeliness of vaccines coadministered with RVV, they have also been associated with a 7 percentage point
lower coverage for RVV compared with these other vaccines.22Although the World Health Organization issued recommendations for loosening this age restriction in developing countries with a higher rotavirus disease burden, no easing of the age
restriction is recommended for lower-burden settings.23Urging earlier DTaP vaccine receipt for infants may be 1 potential way of remediating a missed opportunity for RVV receipt arising from these age restrictions.
NICU and hospital nursery
vaccination practices involving live-attenuated virus vaccines, such as RVV, also appear to have played a role in lower RVV uptake in our cohort.
Children born before 37 weeks’
gestation had 68% lower odds of RVV initiation compared with those born at 37 weeks or later. Many of these preterm children may have had
prolonged stays in a NICU or hospital nurseries and missed vaccination opportunities. A previous study revealed that 63% of infants with very low birth weight failed to receive RVV at the time of NICU discharge often because they exceeded the upper age limit for vaccine receipt.24 A study of a more recent cohort of infants discharged from a NICU revealed that RVV coverage at discharge was 33% vs 83% for other vaccines, with 43% of children without the RVV discharged after the age cutoff.25The shedding of RVV strains has been raised as a potential concern in these settings, but real-world NICU observations have not revealed vaccine-type rotavirus to be detected in infants who are unvaccinated, including those whose NICU stays overlapped by time and location with those of patients who were vaccinated.25,26
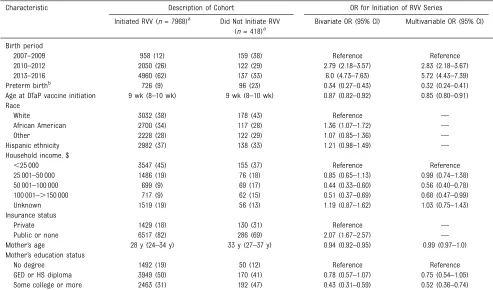
TABLE 1Description of the Cohort and Factors Associated With RVV Series Initiation (NVSN, December 2014–June 2016)
Characteristic Description of Cohort OR for Initiation of RVV Series
Initiated RVV (n= 7968)a Did Not Initiate RVV
(n= 418)a
Bivariate OR (95% CI) Multivariable OR (95% CI)
Birth period
2007–2009 958 (12) 159 (38) Reference Reference
2010–2012 2050 (26) 122 (29) 2.79 (2.18–3.57) 2.83 (2.18–3.67)
2013–2016 4960 (62) 137 (33) 6.0 (4.73–7.63) 5.72 (4.43–7.39)
Preterm birthb 726 (9) 96 (23) 0.34 (0.27–0.43) 0.32 (0.24–0.41)
Age at DTaP vaccine initiation 9 wk (8–10 wk) 9 wk (8–10 wk) 0.87 (0.82–0.92) 0.85 (0.80–0.91)
Race
White 3032 (38) 178 (43) Reference —
African American 2700 (34) 117 (28) 1.36 (1.07–1.72) —
Other 2228 (28) 122 (29) 1.07 (0.85–1.36) —
Hispanic ethnicity 2982 (37) 138 (33) 1.21 (0.98–1.49) —
Household income, $
,25 000 3547 (45) 155 (37) Reference Reference
25 001–50 000 1486 (19) 76 (18) 0.85 (0.65–1.13) 0.99 (0.74–1.38)
50 001–100 000 699 (9) 69 (17) 0.44 (0.33–0.60) 0.56 (0.40–0.78)
100 001–.150 000 717 (9) 62 (15) 0.51 (0.37–0.69) 0.68 (0.47–0.99)
Unknown 1519 (19) 56 (13) 1.19 (0.87–1.62) 1.03 (0.75–1.43)
Insurance status
Private 1429 (18) 130 (31) Reference —
Public or none 6517 (82) 286 (69) 2.07 (1.67–2.57) —
Mother’s age 28 y (24–34 y) 33 y (27–37 y) 0.94 (0.92–0.95) 0.99 (0.97–1.0)
Mother’s education status
No degree 1492 (19) 50 (12) Reference Reference
GED or HS diploma 3949 (50) 170 (41) 0.78 (0.57–1.07) 0.75 (0.54–1.05)
Some college or more 2463 (31) 192 (47) 0.43 (0.31–0.59) 0.52 (0.36–0.74)
Missing data for initiated RVV group: preterm status,n= 24; race,n= 8; ethnicity,n= 10; insurance status,n= 22; mother’s age,n= 42; mother’s education status,n= 64. Missing data for did not initiate RVV group: race,n= 1; ethnicity,n= 1; insurance status,n= 2; mother’s age,n= 3; mother’s education status,n= 6. GED, general equivalency diploma; HS, high school;—, not applicable.
Children born in the years shortly after introduction of RVV had a higher risk of missed RVV initiation. Many reasons may have played a role in thisfinding, including variations in insurance adoption and reimbursement rates,27 caregiver lack of knowledge regarding rotavirus disease,28or hesitancy regarding RVV safety given that the first licensed RVV (RotaShield; Wyeth Laboratories, Marietta, PA) was withdrawn because of concerns regarding intussusception.27,29 Socioeconomic and educational factors differed for RVV initiation versus completion. Children from households with an income of $50 000 to $100 000 had 44% lower odds of initiating RVV compared with children from households with an
income of,$25 000. Mothers with
some college education were half as likely to initiate RVV for their child compared with those who had no high school degree. Interestingly, thesefindings stand in contrast to those of an earlier study of factors associated with RotaShield, which revealed that infants who were from households with a higher economic status, who had mothers with higher education, who were of white race, and who were of non-Hispanic ethnicity were more likely to have initiated RotaShield.30
Our data revealed that once RVV was initiated, income was not significantly associated with RVV completion, and mothers with higher educational attainment were more likely to have their children complete the RVV series. Additionally, African American children and those with public or no insurance had lower odds of series completion compared with white children and those with private insurance, respectively. Whether decreased RVV initiation among wealthier parents with higher education is due to vaccine
hesitancy,31and whether some
wealthier families with greater educational attainment (if they do initiate RVV) are more likely to
complete the series because of greater access to medical care, requires further study.
Geographical variation may have played a role in these discrepancies in RVV initiation and completion. Stratified by geographic location, income remained a significant predictor of RVV initiation in Kansas City, and maternal educational status remained a significant predictor in Oakland. Ethnicity was not associated with RVV initiation in our full sample, as reported elsewhere16; however, site-specific analyses revealed that Hispanic ethnicity doubled the odds of RVV initiation in Seattle and Houston, whereas it halved the odds in Kansas City. For series completion, examples from the literature reveal a mixed picture. An insurance claims–based study of a commercially insured cohort revealed that DTaP
vaccine initiation, visits with a pediatrician versus a family physician, and living in a large urban area versus smaller urban and rural areas predicted RVV completion.17 The authors of a case-control study in Georgia assessed factors associated with RVV series completion and found that non-Hispanic children had a higher odds of an incomplete RVV series, as did children born in earlier birth years and those who did not complete the DTaP vaccine series.16 Additional research is needed to further explore thesefindings.
Our study had several limitations. First, the population used for most analyses included children with rotavirus disease, and although these analyses should not be interpreted as providing standard estimates of vaccine coverage, they are appropriate for comparing RVV and
DTaP vaccine initiation and
completion patterns. Additionally, our subjects were enrolled in surveillance at 7 medical institutions and may not be representative of all US children. Next, we did not collect DTaP vaccine data before 2015, resulting in 1 comparison year. However, the proportions vaccinated in our assessment are similar to
discrepancies between DTaP vaccine and RVV uptake observed elsewhere. RVVs were available at different times in the states where NSVN sites are located and early after vaccine introduction; this likely played a role in coverage. Next, we were unable to separately analyze the group of children with public insurance or no insurance because the sample size was too small; as such, we were unable to evaluate factors associated with RVV receipt among this specific subgroup. Also, we did not have detailed TABLE 3Factors Associated With RVV Series Completion (NVSN, December 2014–June 2016)
Characteristic Description of Cohort OR for Completion of RVV Series
Completed RVV (n= 6101)a Did Not Complete RVV
(n= 1180)a
Bivariate OR (95% CI) Multivariable OR (95% CI)
Birth period
2007–2009 804 (13) 222 (19) Reference Reference
2010–2012 1804 (30) 354 (30) 1.41 (1.17–1.70) 1.37 (1.13–1.67)
2013–2016 3493 (57) 604 (51) 1.60 (1.35–1.90) 1.57 (1.32–1.88)
Preterm birthb 540 (9) 129 (11) 0.79 (0.64–0.96) 0.76 (0.62–0.94)
Age at DTaP vaccine initiation 9 wk (8–10 wk) 9 wk (8–12 wk) 0.94 (0.92–0.95) 0.94 (0.93–0.96)
Race
White 2303 (38) 370 (31) Reference Reference
African American 2059 (34) 445 (38) 0.74 (0.64–0.86) 0.82 (0.70–0.97)
Other 1732 (28) 364 (31) 0.76 (0.65–0.90) 0.84 (0.71–0.99)
Hispanic ethnicity 2275 (37) 453 (38) 0.95 (0.84–1.08) —
Household income, $
,25 000 2725 (45) 576 (49) Reference —
25 001–50 000 1153 (19) 236 (20) 1.03 (0.87–1.22) —
50 001–100 000 560 (9) 83 (7) 1.43 (1.11–1.83) —
100 001–.150 000 534 (9) 62 (5) 1.82 (1.38–2.40) —
Unknown 1129 (19) 223 (19) 1.07 (0.90–1.27) —
Insurance status
Private 1093 (18) 140 (12) Reference Reference
Public or none 4992 (82) 1037 (88) 0.62 (0.51–0.74) 0.75 (0.60–0.93)
Mother’s age 29 y (24–34 y) 28 y (24–33 y) 1.0 (0.99–1.01) —
Mother’s education status
No degree 1137 (19) 250 (22) Reference Reference
GED or HS diploma 3020 (50) 627 (54) 1.06 (0.90–1.25) 1.07 (0.91–1.27)
Some college or more 1898 (31) 282 (24) 1.48 (1.23–1.78) 1.31 (1.07–1.60)
Missing observations for RVV completers:n= 18 for preterm birth;n= 8 for age at DTaP vaccine initiation;n= 25 for mother’s age;n= 7 for race;n= 5 for ethnicity;n= 16 for insurance status;n= 46 for mother’s educational status. Missing observations for RVV noncompleters:n= 9 for preterm birth;n= 5 for age at DTaP vaccine initiation;n= 18 for mother’s age;n= 1 for race;n= 3 for ethnicity;n= 3 for insurance status;n= 21 for mother’s educational status. GED, general equivalency diploma; HS, high school;—, not applicable.
information on premature children and were unable to assess age at hospital discharge or whether they were in a NICU. Finally, we were unable to assess direct caregiver- or provider-related information regarding missed opportunities. As such, we were unable to further explore vaccine hesitancy, which was reported as a possible factor impacting the implementation of rotavirus immunization in the United States.32
CONCLUSIONS
For both the initiation and completion of the ACIP-recommended RVV series, age restrictions and preterm birth were at least partially responsible for lower RVV coverage when compared with that of the DTaP vaccine. Sociodemographic factors played a role, including household income, maternal educational, insurance status, and demographics, all with geographic variation. Timely DTaP vaccine administration may help improve RVV coverage, and further exploration of preterm children and socioeconomic factors may aid in developing public health efforts to improve RVV coverage in the United States.
ACKNOWLEDGMENTS
We thank Samantha H. Johnston for her assistance and the enrollment staff and data managers at each study site.
ABBREVIATIONS
ACIP: Advisory Committee on Immunization Practices AGE: acute gastroenteritis CI: confidence interval
DTaP: diphtheria-tetanus-acellular pertussis
IIS: Immunization Information System
NVSN: New Vaccine Surveillance Network
OR: odds ratio RVV: rotavirus vaccine
Thefindings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and
Prevention.
DOI:https://doi.org/10.1542/peds.2018-1824
Accepted for publication Nov 19, 2018
Address correspondence to Negar Aliabadi, MD, MS, Global Immunization Division, Center for Global Health, US Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H24-2, Atlanta, GA 30329. E-mail: ydh6@cdc.gov
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2019 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:Dr Bernstein received a patent on the GlaxoSmithKline rotavirus vaccine; the other authors have indicated they have nofinancial relationships relevant to this article to disclose.
FUNDING:This article was prepared without any specificfinancial support. The New Vaccine Surveillance System is supported through a cooperative agreement with the Centers for Disease Control and Prevention.
POTENTIAL CONFLICT OF INTEREST:Dr Halasa received vaccines from SanofiPasteur for 1 of her studies and was a consultant for GlaxoSmithKline and Moderna; Dr Englund reports research support to her institution for clinical studies by GlaxoSmithKline and Merck; Dr Bernstein has received research funding from GlaxoSmithKline, Merck, and Wyeth Laboratories; Dr Harrison was an investigator on research projects for which his institution received grant funds from Merck,
Pfizer, Cubist, Allergan, Regeneron, Janssen, and GlaxoSmithKline; he also received travel funds and an honorarium to present scientific data to Pfizer’s research
team at their corporate headquarters; the other authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
1. Aliabadi N, Tate JE, Haynes AK, Parashar UD; Centers for Disease Control and Prevention (CDC). Sustained decrease in laboratory detection of rotavirus after implementation of routine
vaccination—United States, 2000-2014.
MMWR Morb Mortal Wkly Rep. 2015;
64(13):337–342
2. Payne DC, Staat MA, Edwards KM, et al; New Vaccine Surveillance Network (NVSN). Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties,
2006-2009.Clin Infect Dis. 2011;53(3):245–253
3. Leshem E, Moritz RE, Curns AT, et al. Rotavirus vaccines and health care utilization for diarrhea in the United
States (2007-2011).Pediatrics. 2014;
134(1):15–23
4. Cortese MM, Parashar UD; Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children:
recommendations of the Advisory Committee on Immunization Practices (ACIP) [published correction appears in
MMWR Recomm Rep. 2010;59(33):1074].
MMWR Recomm Rep. 2009;58(RR-2):
1–25
5. Robinson CL; Advisory Committee on Immunization Practices (ACIP), ACIP Child/Adolescent Immunization Work Group. Advisory Committee on
Immunization Practices recommended immunization schedules for persons
aged 0 through 18 years–United States,
2016.MMWR Morb Mortal Wkly Rep.
2016;65(4):86–87
6. Strategic Advisory Group of Experts on Immunization. Meeting of the Strategic Advisory Group of Experts on
immunization, April 2012–conclusions
and recommendations.Wkly Epidemiol
Rec. 2012;87(21):201–216
7. Centers for Disease Control and Prevention (CDC). National, state, and local area vaccination coverage
among children aged 19-35 months—
United States, 2009.MMWR Morb
Mortal Wkly Rep. 2010;59(36):1171–1177
8. Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19-35
months - United States, 2014.MMWR
Morb Mortal Wkly Rep. 2015;64(33):889–896
9. Elam-Evans LD, Yankey D, Singleton JA, Kolasa M; Centers for Disease Control and Prevention (CDC). National, state, and selected local area vaccination coverage among children aged 19-35
months - United States, 2013.MMWR
Morb Mortal Wkly Rep. 2014;63(34):
741–748
10. Centers for Disease Control and Prevention (CDC). National, state, and
local area vaccination coverage among
children aged 19-35 months–United
States, 2011.MMWR Morb Mortal Wkly
Rep. 2012;61:689–696
11. Centers for Disease Control and Prevention (CDC). National, state, and local area vaccination coverage among children aged 19-35 months - United
States, 2012.MMWR Morb Mortal Wkly
Rep. 2013;62(36):733–740
12. Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Dietz V. Vaccination coverage among children aged
19-35 months - United States, 2015.MMWR
Morb Mortal Wkly Rep. 2016;65(39):
1065–1071
13. Pringle K, Cardemil CV, Pabst LJ, Parashar UD, Cortese MM. Uptake of rotavirus vaccine among US infants at immunization information system
sentinel sites.Vaccine. 2016;34(50):
6396–6401
14. Gebremeskel BG, Zhang D, Goveia MG,
Marshall GS, O’Brien MA. Vaccine
coverage for United States infants at milestone ages: missed opportunities
for vaccination.J Pediatric Infect Dis
Soc. 2016;5(4):473–475
Hum Vaccin Immunother. 2016;12(5):
1235–1243
16. Parker TC, Mohammed A, Leong T, et al. Rotavirus vaccination rate disparities seen among infants with acute
gastroenteritis in Georgia.Ethn Health.
2017;22(6):585–595
17. Panozzo CA, Becker-Dreps S, Pate V, et al. Patterns of rotavirus vaccine uptake and use in privately-insured US
infants, 2006-2010.PLoS One. 2013;8(9):
e73825
18. Payne DC, Staat MA, Edwards KM, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States.
Pediatrics. 2008;122(6):1235–1243
19. Food and Drug Administration. Rotarix vaccine: update to clinicians and public health professionals. 2010. Available at: https://wayback.archive-it.org/7993/ 20170112165645/http://www.fda.gov/Sa fety/MedWatch/SafetyInformation/Safe tyAlertsforHumanMedicalProducts/u cm205640.htm
20. Aliabadi N, Tate JE, Parashar UD. Potential safety issues and other factors that may affect the introduction
and uptake of rotavirus vaccines.Clin
Microbiol Infect. 2016;22(suppl 5):
S128–S135
21. Centers for Disease Control and Prevention (CDC); Advisory Committee
on Immunization Practices (ACIP). Rotavirus vaccination coverage and adherence to the Advisory Committee on Immunization Practices (ACIP)-recommended vaccination
schedule–United States, February
2006-May 2007.MMWR Morb Mortal
Wkly Rep. 2008;57(15):398–401
22. Hull BP, Menzies R, Macartney K, McIntyre PB. Impact of the introduction of rotavirus vaccine on the timeliness of other scheduled vaccines: the
Australian experience.Vaccine. 2013;
31(15):1964–1969
23. Rotavirus vaccines. WHO position
paper–January 2013.Wkly Epidemiol
Rec. 2013;88(5):49–64
24. Stumpf KA, Thompson T, Sánchez PJ. Rotavirus vaccination of very low birth weight infants at discharge from the
NICU.Pediatrics. 2013;132(3). Available
at: www.pediatrics.org/cgi/content/full/ 132/3/e662
25. Hofstetter AM, Lacombe K, Klein EJ, et al. Risk of rotavirus nosocomial spread after inpatient pentavalent
rotavirus vaccination.Pediatrics. 2018;
141(1):e20171110
26. Hiramatsu H, Suzuki R, Nagatani A, et al. Rotavirus vaccination can be
performed without viral dissemination in the neonatal intensive care unit.
J Infect Dis. 2018;217(4):589–596
27. Kempe A, Patel MM, Daley MF, et al. Adoption of rotavirus vaccination by pediatricians and family medicine physicians in the United States.
Pediatrics. 2009;124(5). Available at: www.pediatrics.org/cgi/content/full/ 124/5/e809
28. Patel MM, Janssen AP, Tardif RR, Herring M, Parashar UD. A qualitative
assessment of factors influencing
acceptance of a new rotavirus vaccine among health care providers and
consumers.BMC Pediatr. 2007;7:32
29. O’Leary ST, Parashar UD, Crane LA,
et al. Adoption of rotavirus vaccine by U.S. physicians: progress and
challenges.Am J Prev Med. 2013;44(1):
56–62
30. Smith PJ, Schwartz B, Mokdad A, Bloch
AB, McCauley M, Murphy TV. Thefirst
oral rotavirus vaccine, 1998-1999: estimates of uptake from the National
Immunization Survey.Public Health Rep.
2003;118(2):134–143
31. Siddiqui M, Salmon DA, Omer SB. Epidemiology of vaccine hesitancy in
the United States.Hum Vaccin
Immunother. 2013;9(12):2643–2648
32. Lo Vecchio A, Liguoro I, Dias JA, et al. Rotavirus immunization: global coverage and local barriers for
implementation.Vaccine. 2017;35(12):
DOI: 10.1542/peds.2018-1824 originally published online January 17, 2019;
2019;143;
Pediatrics
Umesh D. Parashar and Daniel C. Payne
Moffatt, Christopher J. Harrison, Leila C. Sahni, Laura S. Stewart, David I. Bernstein,
Rangaraj Selvarangan, Janet A. Englund, Parvin H. Azimi, Eileen J. Klein, Mary E.
Boom,
Szilagyi, Mary Allen Staat, Geoffrey A. Weinberg, Natasha B. Halasa, Julie A.
Negar Aliabadi, Mary E. Wikswo, Jacqueline E. Tate, Margaret M. Cortese, Peter G.
Factors Associated With Rotavirus Vaccine Coverage
Services
Updated Information &
http://pediatrics.aappublications.org/content/143/2/e20181824 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/143/2/e20181824#BIBL This article cites 31 articles, 5 of which you can access for free at:
Subspecialty Collections
_sub
http://www.aappublications.org/cgi/collection/vaccine:immunization Vaccine/Immunization
http://www.aappublications.org/cgi/collection/epidemiology_sub Epidemiology
b
http://www.aappublications.org/cgi/collection/infectious_diseases_su Infectious Disease
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2018-1824 originally published online January 17, 2019;
2019;143;
Pediatrics
Umesh D. Parashar and Daniel C. Payne
Moffatt, Christopher J. Harrison, Leila C. Sahni, Laura S. Stewart, David I. Bernstein,
Rangaraj Selvarangan, Janet A. Englund, Parvin H. Azimi, Eileen J. Klein, Mary E.
Boom,
Szilagyi, Mary Allen Staat, Geoffrey A. Weinberg, Natasha B. Halasa, Julie A.
Negar Aliabadi, Mary E. Wikswo, Jacqueline E. Tate, Margaret M. Cortese, Peter G.
Factors Associated With Rotavirus Vaccine Coverage
http://pediatrics.aappublications.org/content/143/2/e20181824
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.