Protein and fat utilization in lactating sows:
I. Effects on milk production and body composition
1J. P. McNamara*
2and J. E. Pettigrew†
*Department of Animal Sciences, Washington State University, Pullman 99164-6351 and †Department of Animal Sciences, University of Illinois, Urbana 61801
ABSTRACT: In order to provide data with which to
challenge a model of metabolism of lactating sows, we conducted a study to determine milk production and body and mammary composition in sows consuming a range of energy and amino acid intakes and nursing 11 to 12 pigs. Sows (2nd through 4th parity) consumed the same ration during gestation and consumed 6.1 kg/ d (as-fed) for a 20 d lactation. Litter size was standard-ized at 12 pigs within 3 d of farrowing. Diets were formulated to provide three different amounts of pro-tein intake and two different amounts of fat intake. Protein intakes of sows in high (HP), medium (MP), and low protein (LP) treatment groups were 863, 767, and 678 g/d with 59, 53, and 47 g/d lysine at two levels of fat intake, 117 (LF) and 410 g/d (HF). Number of pigs weaned per litter was 11.4±0.5 and milk produc-tion and litter weight gain was less (P<0.01) in the last
Key Words: Amino Acids, Fats, Lactation, Metabolism, Simulation Models, Sows
2002 American Society of Animal Science. All rights reserved. J. Anim. Sci. 2002. 80:2442–2451
Introduction
Ability to predict performance of lactating sows re-quires quantitative knowledge of the chemical intercon-versions of amino acids, fatty acids, and glucose in body tissues (Black et al., 1986; Pettigrew et al., 1992a,b; Baldwin, 1995). This knowledge is limited for many biochemical pathways (McNamara and Boyd, 1998). Body fat and body protein are mobilized to meet the needs of lactation, especially when intake of energy and amino acids is limited (Pettigrew et al., 1992a,b; Parmley and McNamara, 1996). Information on the
1College of Agriculture and Home Economics Research Center, Washington State University, Pullman. Project Numbers 0107, 0249, and 3407. Supported in part by the National Institutes of Health (RD24529) and the National Pork Producers Council (1598).
2Correspondence: 233 Clark Hall, P.O. Box 646351, Washington State University, Pullman WA 99164-6351. Phone: 509 335-4113; Fax: 509-335-4246; E-mail: mcnamara@wsu.edu.
Received June 1, 2001. Accepted May 14, 2002.
2442
week of lactation for sows consuming the least protein. Medium and low protein intakes increased (P < 0.05) loss of body lean and protein. Change in carcass protein during lactation was −1.4, −3.0, −2.2, −1.2, −1.9 and
−2.1 kg (SD 2.6) for sows fed HPLF, MPLF, LPLF, HPHF, MPHF, and LPHF. Body fat (carcass and vis-ceral) change was 0.4,−3.7,−4.1,−0.3, 3.4, and−1.3 kg (SD 6.6) in HPLF, MPLF, LPLF, HPHF, MPHF, and LPHF groups. Total amount of mammary parenchyma increased more (P<0.05) in sows fed a higher fat diet. These data are consistent with general knowledge of changes in body composition in lactation of sows. How-ever, changes in body protein and fat were correlated across treatments and different from that reported for sows nursing smaller litters. These data help our quan-titative understanding of nutrient flux in sows nursing large litters and allow a severe challenge of existing models of metabolism in sows.
partitioning of amino acids between milk production and body protein is limited, though more recent reports are addressing this lack (Trottier et al., 1997; Clowes et al., 1998; Dourmad et al., 1998).
Rates of body fat synthesis and mobilization are sen-sitively related to energy intake and milk energy output (Parmley and McNamara, 1996). The recent National Research Council Requirements of Swine (NRC, 1998) suggested an increased requirement for protein and lysine in sows producing large amounts of milk. Since this study was conducted (preliminary results in McNa-mara, 1998) others have also probed the effects of nutri-ent intake on body tissue use (Clowes et al., 1998; Dour-mad et al., 1998; Jones and Stahly, 1999), mammary growth (Kim et al., 1999, 2001a), and mammary amino acid use (Cooper et al., 2001a,b; Kim et al., 2001b). Such data are useful but often not sufficient to set specific parameters for the next generation of mechanistic mod-els (Pettigrew et al., 1992a,b); also, obviously, they were not available when the model of Pettigrew et al. (1992a,b) was constructed nor when this trial was be-gun. Objectives were to 1) determine effects of a range
of protein and fat intakes on body composition and milk production in sows nursing 11 to 12 piglets and 2) to collect data sufficient for challenging behavior and sen-sitivity of an existing mechanistic, dynamic model of metabolism in the lactating sow (Pettigrew et al., 1992a,b).
Materials and Methods
Experimental Procedures
Sows were Landrace × Yorkshire crossbreds and blocked into two parity groups: parity 2 or parity (3 or 4). They were bred by artificial insemination and fed 1.84 kg as fed of the same diet (corn/soybean; 13.5% CP, 3.3 Mcal ME/kg as fed), balanced to NRC require-ments for restricted gestation feeding (NRC, 1988). The lactation feeding experiment was designed as a ran-domized complete block, with two levels of dietary fat and three levels of dietary protein and parity as block. Primiparous animals were not used, since their metabo-lism and production are significantly different from older animals, and animals of parity greater than 4 were not used since we had a limited number in our herd and we wished to minimize extraneous variation. From farrowing onward, sows were fed twice daily (be-tween 0700 and 0900 and 1600 and 1800) the following diets (all percentages as fed): Low Protein, Low Fat, (LPLF): 2% added fat, 11.6% CP, 0.8% lysine; Medium Protein, Low Fat (MPLF): 2% added fat, 13.1% CP, 0.9% lysine; High Protein, Low Fat (HPLF): 2% added fat, 14. 7% CP, 1.0% lysine; Low Protein, High Fat (HPHF): 7% added fat, 11.6% CP, 0.8% lysine; Medium Protein, High Fat (MPHF): 7% added fat, 13.1% CP, 0.9% lysine; and High Protein, High Fat (HPHF): 7% added fat, 14.7% CP, 1.0% lysine (Table 1). These diets were formulated to either meet (LP) or exceed (MP,
HP) the amounts recommended as required by the NRC in 1988. There were 18 animals assigned to each treat-ment group, resulting in 54 in each fat intake group and 36 in each protein intake group. Diets were offered up to 5.5 kg/d (for a given individual animal) as fed during wk 1 and 6.8 kg/d for wk 2 and 3. Feed offerings were adjusted downwards for individual animals if they did not consume all the feed offered; however, animals usually consumed all that was fed. In those few in-stances when all food offered was not consumed, orts were collected, weighed, and actual feed intake deter-mined. There were several reasons for setting treat-ments this way. By early 1995, when this experiment was designed, it was already recognized that the recom-mendations in the 1988 guide were most likely too low for more modern sow genotypes nursing larger litters. We wanted to specifically test effects of total protein and fat (not specific amino acids or glucose (from starch)); we wanted to feed protein above the recommended level to sows nursing large litters. Our herd records indicated our sows voluntarily consumed more feed than the aver-ages given in the 1988 NRC, and we wanted to provide
data for a challenge of a metabolic model (Pettigrew et al., 1992a) that was constructed using data from sows, which were in general consuming less feed and nursing smaller litters than the present sows. Day 1 of lactation is first full 24-h period after farrowing, starting at 0700 each calendar day.
Sampling Regimen
Litters were adjusted to 12 pigs within 3 d of parturi-tion. There was no minimal or maximal cut-off for litter sizes for use of sows, and in practice the smallest litter size was three, and the largest was 16. Sow body weights were taken 5 d prepartum and weekly postpar-tum. Ultrasound measurements of subcutaneous fat over the 10th and last ribs (65 mm lateral to the dorsal midline) were conducted using real-time ultrasound [Aloka 210 B-mode, Corometrics Medical Systems, Wallingford, CT; Animal Ultrasound Services, Inc., Ith-aca, NY; and Superflab (cat # 8117) Mick Radio-Nuclear Instrumentation, New York, NY; with a wide view field transducer (U5T-5021-3)]. Litters were weighed twice weekly (Tuesdays and Fridays). Milk samples were taken on d 7 and 20 of lactation. After removal of the litter for 1 h, an injection of 0.5 units of oxytocin was given, followed by reintroduction of half of the litter to induce milk letdown and allow sampling of glands. Ev-ery attempt was made to sample from three or more mammary glands; this was not possible for every sow at every milking, yet this was the overwhelming norm. Milk production was calculated from litter weights and growth rates following equations in (Noblet and Etienne, 1989 [milk (kg)=(2.50×ADG)+(80.2×piglet BW)+7]). Milk DM and ash were determined by stan-dard methodologies (AOAC, 1990), fat was extracted in chloroform: methanol (Folch et al., 1957), and milk protein was determined by the dyebinding method of Bradford (1976).
Body Composition
Fifteen sows were killed at d 1 of lactation for a com-parative slaughter group (initial slaughter (IS)). They were allotted to this group randomly, and contemporar-ily with sows going on feeding treatments. At end of treatment, six animals from each treatment were killed at d 21 for body composition. There were no restrictions on selection of IS group or slaughter sows; it was a random allotment. Sows were killed under USDA guidelines by captive bolt stunning followed by exsan-guination. Blood and organs were collected and weighed (gastrointestinal contents were not removed because animals were fasted overnight and we were not cerned with variation due to remaining intestinal con-tents). The right half of each carcass dissected (after an overnight chilling at 4°C) into trimmable fat, lean, and bone. Separation of fat from lean was such that upon visual appraisal, in the fat there were only very minor and small flecks of lean; bone was trimmed of
Table 1. Composition of diets varying in protein and fat fed to lactating sows
Low fat High fat
Component LPa MP HP LP MP HP % as-fed Barley 91.7 87.6 83.1 85.1 81.0 76.9 Soybean meal, 48 2.10 6.30 10.80 3.80 7.90 12.00 Lysine, 78.3% 0.47 0.45 0.43 0.44 0.43 0.41 Animal fat 2.00 2.00 2.00 7.00 7.00 7.00 Limestoneb 1.00 1.00 1.00 1.00 1.00 1.00
Calcium phosphate, dibasicb 1.40 1.40 1.40 1.40 1.40 1.40
NaCl, iodizedb 0.50 0.50 0.50 0.50 0.50 0.50
Trace mineral, w/o Seb 0.05 0.05 0.05 0.05 0.05 0.05
Sow vitamin mixc 0.50 0.50 0.50 0.50 0.50 0.50
Selenium premixb 0.05 0.05 0.05 0.05 0.05 0.05
TM-50b 0.05 0.05 0.05 0.05 0.05 0.05
Mg sulfateb 0.15 0.15 0.15 0.15 0.15 0.15
Chemical composition, calculated, as-fed
ME, Mcal/kg 3.01 3.02 3.03 3.29 3.29 3.30
Crude protein, % 11.6 13.1 14.7 11.6 13.1 14.6
Lysine, % 0.80 0.90 1.00 0.80 0.90 1.00
aHP=high protein, MP=medium protein, and LP=low protein. Low fat was 2% added fat; high fat was 7% added fat.
bDicalcium phosphate: 20 to 24% Ca, 18.5% P minimum, 0.185% F, maximum; limestone: 37 to 39% Ca; NaCl includes 0.01% I as ethylene diamine dihydroiodide; selenium premix: 0.02% Se (Na selenite) and 36 to 39% Ca; TM-50: oxytetracycline 110 g/kg; trace mineral: in limestone, no cobalt, 10 to 12% Ca, 10% Mn, 10% Fe, 10% Zn, 1% Cu, 0.03% I; Mg sulfate: 9.8% Mg.
cSow vitamin mix: 880,000 IU vit. A/kg; 66,000 IU vit. D3/kg; 4,400 IU vit. E/kg, 792 mg vit. B
2/kg: 2,640 mg niacin/kg, 2,640 mgd-pantothenate/kg, 99,000 mg choline/kg, 3.96 mg B12/kg; 198 mg menadione sodium bisulfite/kg; 220 mg folic acid/kg; 19.8 mgd-biotin/kg; 9,988 mg ethoxyquin/kg.
lean and fat and any bits of lean or fat remaining on bone after first cutting were trimmed and placed in the appropriate sample. Lean was ground thorough a 0.95 cm opening plate, mixed, and sampled. Chemical dry matter and ash were determined by AOAC techniques (AOAC, 1990). Fat content of the lean was extracted in chloroform: methanol (Folch et al., 1957). Muscle pro-tein content was calculated as fat-free lean multiplied by 0.207 (Pettigrew et al., 1992a,b). To calculate the change in body protein and fat during lactation, the average percentage of body fat and protein of BW from the IS group was multiplied by the actual individual BW of the sows used for chemical analysis at the end of lactation. Change in body fat or protein was then determined as the difference of the individual sow end-ing composition and the individual sow initial compo-sition.
Mammary glands were collected in total and weighed. The same mammary gland (4th from first inguinal gland, right side; if this gland was not functional, the next gland caudally was used) was excised, weighed, and dissected into skin, fat, lean, and parenchyma. Pa-renchyma only was extracted for analysis of DNA (Ken-singer et al., 1982).
Experimental Design and Analysis
The production experiment was designed as a ran-domized complete block with repeated measures in time, with parity (2 or 3 and 4) as blocks. The composi-tion aspect was a randomized complete block with a
split-plot in time of different experimental units at each time point (comparative slaughter). Factorial treat-ments of three protein levels and two fat levels were applied to groups of 18 sows. For production and compo-sition data, main and interaction effects of parity, pro-tein intake, fat intake, and their interaction (propro-tein× fat; parity×protein; parity×fat; parity×protein×fat) were determined against the error mean square for sow within (parity×fat×protein) using the appropriate F statistic (Steel and Torrie, 1980). If the parity×protein
×fat interaction was not significant (it almost always was not), it was pooled into the error term. Therefore, the model without that effect was usually the final model for all variables, unless stated otherwise. For measures where an initial value was available (e.g., sow body weight, composition, litter live birth weight), the initial value was tested as a covariable, and if this effect was significant, that analysis was used. Effects were considered significant if P< 0.05, although in a few instances we have reported effects with P value between 0.05 and 0.10 and discussed them separately. In an additional set of analyses with the objective of describing some empirical relationships between ob-served or simulated uses of body nutrients, variables of body composition (body fat, lean, protein, change in lean, fat, and protein) were regressed on each other (Steel and Torrie, 1980). Linear, quadratic, and loga-rithmic regressions were tested; however, for all vari-ables the best fit of data were provided by the linear regression only. Calculations were done using PROC GLM and PROC REG of SAS (SAS Inst. Inc., Cary, NC).
Table 2. Feed intake and body weights of lactating sows fed diets varying in protein and energy
Low fata High fat
Item LP MP HP LP MP LP SD ADFIb, kg/d as fed Week 1 5.2 5.3 5.2 5.2 5.2 5.3 0.5 Week 2 6.3 6.5 6.4 6.5 6.3 6.4 0.5 Week 3 6.2 5.8 6.3 5.6 6.3 5.9 1.1 CP intake, g/d 683 769 879 671 782 862 31 No. 18 18 17 20 19 18 Sow BW, kg Day−5 220 224 226 228 228 237 17.9 Day 1 211 216 212 215 219 229 16.9* Day 7 205 213 221 216 219 229 16.8* Day 14 199 206 214 211 215 225 17.4* Day 20 188 197 206 200 207 220 17.6* Change in BW, d 1 to d 20, kg −23 −19 −6 −15 −12 −9 10.6* aHP=high protein, MP=medium protein, and LP=low protein. Low fat was 2% added fat; high fat was 7% added fat.
bAverage daily feed intake. There were no significant treatment effects on average daily feed intake, as planneda priori.
*BW was analyzed with BW at d 1 as covariable. Covariable for all BW measures (P<0.05). There was an effect of fat (P<0.03) for change in BW and for BW at d 20; for d 7 and d 14 (P=0.10). There was a main effect of protein intake (P<0.02) for BW at d 7, 14, and 20 and for change in BW.
Results and Discussion
Intake of diets was similar among treatments, as planned, with no difference (P > 0.10) in feed intake during lactation (Table 2). Covariate adjusted (d 1 value as covariate) sow body weights at 20 d were lower (P
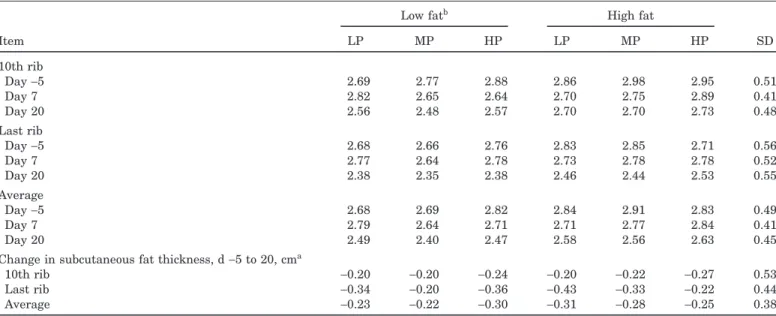
<0.03) and body weight loss was greater (P<0.03) for sows consuming low fat rations (Table 2). There was a main effect of protein intake (P<0.02) for BW at d 7, 14, and 21 and for change in BW, with the sows consum-ing the highest protein losconsum-ing less BW. There was no effect (P > 0.10) of energy or protein intake on sow backfat thickness nor changes in backfat thickness (Ta-ble 3).
Litters gained 50.7 kg (SE = 0.73 kg, n = 108) on average over the 20 d (20.3 d±0.09, n=108; range 17 to 22 d) lactation. Those sows consuming the lowest protein tended to produce a lower litter gain (P<0.08) than the other groups (Table 4). The reduction in litter growth came primarily in the last week of lactation (Table 4). This is still one of the highest rates of litter gain for sows reported in the scientific literature (refer to any of the several references listed), demonstrating the milking capacity of these animals and providing a severe challenge for model behavior. The decrease in piglet growth due to low protein intake of the sow com-pared to the average of medium and high protein was 2 kg per litter for the last 3 d of lactation. There was neither a main effect of fat nor an interaction of fat and protein (P>0.10).
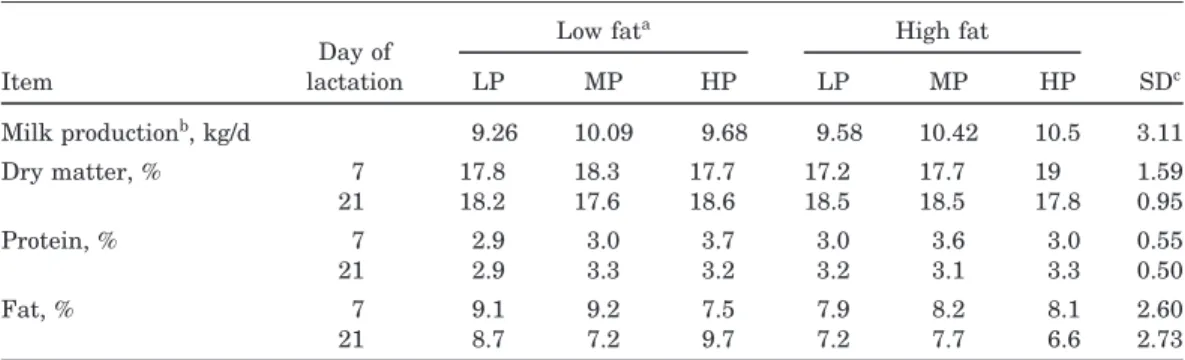
Milk dry matter percent was unaffected (P>0.10) by dietary protein intake (Table 5). There was an interac-tion of protein and fat intake on milk protein percent-age, such that milk protein percentage decreased with
decreasing protein intake on the low-fat diet (P<0.02), but not on the high-fat diet. Milk protein percentage and milk DM in this study were lower than is usually reported for sow milk (King et al., 1993; Dourmad et al., 1998; Pluske et al., 1998). There may be a method-ological difference in that we used a soluble protein dyebinding assay to measure true protein, whereas many researchers analyze milk total N and use a multi-plier for protein. Total N usually overestimates true protein in milk approximately 0.2 percentage units. This alone would not account for the low protein values, which were measured about 2.9 to 3.7%, while other measures have been above 5%. Another potential hy-pothesis is that because these animals were nursing almost 12 pigs for 20 d and producing a large volume of milk (one of the largest reported) is that the milk of these sows was indeed lower in protein. However, when Dourmad et al. (1998) reported data on sows nursing 11 to 12 pigs, the protein percentage in milk from those sows was close to 5% (they measured total milk N, and whether using a multiplier of 6.38, 6.25, or 5.83 (porcine muscle), the milk protein would be still be about 5%). However, their reported milk production was 1.5 to 2.5 kg per d less than the sows reported here and they determined milk yield with the same method as we used. Renaudeau and Noblet (2001) reported similar milk yields to the present ones but a greater protein percentage. Thus, although there may be a genetic dif-ference in this herd or a production level difdif-ference, the low protein values were most likely due to the technique used. For this reason we are not making any inferences of treatment effects on milk protein in this study (the available literature would suggest that at these protein intakes, a large change in protein composition might
Table 3. Subcutaneous fat thickness (cm)aof sows fed varying amounts of protein and fat during lactation
Low fatb High fat
Item LP MP HP LP MP HP SD 10th rib Day−5 2.69 2.77 2.88 2.86 2.98 2.95 0.51 Day 7 2.82 2.65 2.64 2.70 2.75 2.89 0.41 Day 20 2.56 2.48 2.57 2.70 2.70 2.73 0.48 Last rib Day−5 2.68 2.66 2.76 2.83 2.85 2.71 0.56 Day 7 2.77 2.64 2.78 2.73 2.78 2.78 0.52 Day 20 2.38 2.35 2.38 2.46 2.44 2.53 0.55 Average Day−5 2.68 2.69 2.82 2.84 2.91 2.83 0.49 Day 7 2.79 2.64 2.71 2.71 2.77 2.84 0.41 Day 20 2.49 2.40 2.47 2.58 2.56 2.63 0.45
Change in subcutaneous fat thickness, d−5 to 20, cma
10th rib −0.20 −0.20 −0.24 −0.20 −0.22 −0.27 0.53
Last rib −0.34 −0.20 −0.36 −0.43 −0.33 −0.22 0.44
Average −0.23 −0.22 −0.30 −0.31 −0.28 −0.25 0.38
aFat thickness measures analyzed with d−5 value as covariable. There were no effects (P>0.05) of protein, fat, or parity on fat thickness measures.
bHP=high protein, MP=medium protein, and LP=low protein. Low fat was 2% added fat; high fat was 7% added fat.
not be expected), and the effect on errors in model chal-lenges is discussed in that section.
Sow body composition during lactation changed as expected from previous knowledge on use of dietary protein and fat (Tables 6 and 7) with a few exceptions. Most internal organ weights did not change appreciably over the course of lactation or due to treatment. Total body fat was gained in groups consuming low fat with high protein and high fat with either medium or high protein and lost in other treatment groups; however, these trends had only aP=0.11 for protein main effect and 0.13 for fat main effect. The old sorry story of “not enough animals to test the hypothesis” probably applies
Table 4. Weight and growth of litters from sows fed varying dietary protein and fat during lactation
Day of lactation
d 1 to 20a
Dietary treatment 1 4 7 10 14 17 21 (wt change)
Litter weight, kg Low fatb LP 20.4 24.5 33.0 42.2 51.4 60.5 69.1 48.7 MP 21.7 27.0 34.7 44.0 54.0 64.4 74.2 52.6 HP 20.4 25.3 32.7 41.8 50.9 62.2 71.9 51.5 High fat LP 21.1 25.3 32.7 42.6 52.1 63.5 69.9 48.8 MP 19.9 25.1 34.1 43.4 54.5 64.1 72.5 52.6 HP 20.0 24.7 30.5 39.7 49.4 60.1 70.2 50.2 SD 4.1 4.0 5.8 6.2 7.3 7.5 8.3 7.8+
aChange in litter weight (kg) from d 1 to d 20.
bHP=high protein, MP=medium protein, and LP=low protein. Low fat was 2% added fat; high fat was 7% added fat.
+Dietary protein effect (P<0.08) on litter growth from d 1 to 20 andP<0.09 on growth from d 17 to 20,
the latter only using initial litter weight as covariable.
here, but the data also illustrate the larger problem of the number of animals it now takes to measure signifi-cant treatment differences in important traits.
Trimmed carcass lean and protein decreased during lactation in all groups and the loss was increased (P<
0.05) if the sows consumed the LP rations. The amount of dietary protein intake (approx. 800 g/d) below which sows lost body protein is greater in relation to suggested requirements in the 1988 NRC guidelines; however, it is in good agreement with the newer revision (NRC, 1998). Sows consuming the medium dietary protein amount (740 to 780 g/d) did lose more loin eye area but not more protein than those consuming the HP diet.
Table 5. Composition of milk from sows fed varying protein and fat during lactation
Low fata High fat Day of Item lactation LP MP HP LP MP HP SDc Milk productionb, kg/d 9.26 10.09 9.68 9.58 10.42 10.5 3.11 Dry matter, % 7 17.8 18.3 17.7 17.2 17.7 19 1.59 21 18.2 17.6 18.6 18.5 18.5 17.8 0.95 Protein, % 7 2.9 3.0 3.7 3.0 3.6 3.0 0.55 21 2.9 3.3 3.2 3.2 3.1 3.3 0.50 Fat, % 7 9.1 9.2 7.5 7.9 8.2 8.1 2.60 21 8.7 7.2 9.7 7.2 7.7 6.6 2.73
aHP=high protein, MP=medium protein, and LP=low protein. Low fat was 2% added fat; high fat was 7% added fat.
bMilk production was calculated from litter weight and litter growth using the following equation from Noblet and Etienne (1989) for d 1 to 20, milk (kg)=7+(2.50×ADG)+(80.2×piglet BW), where weights are in kg on a per pig basis. This gives the daily per pig milk production, if multiplied by the number of pigs and the number of days.
cFor protein percent, interaction of protein and fat (P<0.06).
Recently, Yang et al. (2000) reported that sows consum-ing 20 g/d of lysine and 11.9% CP had an increase in the fractional breakdown rate of muscle protein, which would be consistent with the intake and body composi-tion data in this experiment. The loss of muscle protein in the LP fed group was noted in sows consuming either amount of fat. There were no interactions of protein by
Table 6. Body composition of sows entering lactation and of sows fed varying amounts of protein and fat during lactation and used in comparative slaughter analysis
Low fata High fat
Item ISb LP MP HP LP MP HP SD Effect* Body weight, kg 216.2 184.5 192.3 204.6 191.3 200.5 220.8 23.0 P Blood, kg 7.70 6.7 7.1 7.8 7.1 7.4 8.4 1.2 Head, kg 14.80 13.6 13.8 14.5 14.1 14.4 15.4 1.7 Feet, kg 2.91 2.77 3.01 2.82 2.87 3.03 3.30 0.42 Skin, kg 18.5 16.4 16.6 18.0 16.1 17.8 19.9 3.1 Liver, kg 3.95 2.87 3.03 2.83 3.02 2.87 3.31 0.43 Gall bladder, kg 0.16 0.20 0.29 0.24 0.16 0.27 0.21 0.08 Heart, kg 0.71 0.61 0.65 0.63 0.62 0.63 0.71 0.10 Spleen, kg 0.34 0.22 0.35 0.31 0.27 0.30 0.32 0.09 Lungs, kg 2.12 2.05 2.43 2.37 2.21 2.41 2.96 0.51 Kidneys, kg 0.43 0.38 0.40 0.48 0.42 0.43 0.49 0.08
Stomach and esophagus, kg 6.47 3.99 3.81 2.99 4.30 3.13 2.66 1.73 Small intestine, kg 5.88 5.57 6.15 5.27 6.10 5.57 7.14 1.29 Large intestine, kg 7.14 9.59 9.61 9.73 9.80 9.47 9.57 1.83 Reproductive tract, kg 4.66 1.40 1.55 1.60 1.39 1.25 1.38 0.53 Abdominal fat, kg 2.88 2.08 2.89 3.05 2.15 2.99 3.09 0.80 P Mammary glands, kg 10.26 8.37 9.99 10.28 10.30 9.73 10.95 1.55 P Hot carcass wt, kg 124.1 104.7 110.0 117.7 107.1 112.9 127.0 14.7 P Cold carcass wt, kg 119.5 100.1 103.9 113.7 106.4 109.8 123.6 14.7 P, F Loin eye area, cm2 114.6 92.0 91.2 103.5 92.0 95.9 118.3 18.1 P
Last rib backfat, cm 2.02 1.74 2.18 1.99 1.63 2.22 1.93 0.53 P
10th-rib backfat, cm 2.78 2.33 2.41 2.71 2.72 3.22 2.46 0.78
Bone, kg 22.6 21.6 22.7 19.2 21.6 21.1 23.6 3.2 P×F
Trimmed fat, kg 16.9 15.2 15.3 19.2 15.7 20.2 18.2 5.5
Trimmed lean, kg 81.6 66.4 68.6 76.6 69.4 70.9 84.2 11.0 P, F
Total fat, kg 19.7 17.2 18.1 22.2 17.9 23.2 21.3 6.0
(sum abdominal and trim)
aHP=high protein, MP=medium protein, and LP=low protein. Low fat was 2% added fat, high fat was 7% added fat.
+Effect definitions are P=protein main effect, F=fat main effect, P×F=interaction, all atP<0.05 or lower.
bInitial slaughter: Sows killed between d 1 and d 3 of lactation for initial comparison group.
fat in any body composition variables measured except bone, and we have no explanation for this effect.
Several regressions were conducted to explore the relationship between changes in body components. Con-ducting this type of experiment over a wide range of protein intakes allows exploration of some relation-ships, which are relatively still undefined, especially in
Table 7. Chemical composition of trimmed lean of sows fed varying amounts of protein and fat during lactation
Low fata High fat
Item ISb LP MP HP LP MP HP SD Effectc LBW 216.2 184.5 192.3 204.6 191.3 200.5 220.8 23.0 P, F Lean, kg 81.6 66.4 68.6 76.6 69.4 70.9 84.2 11.0 P, F Fat in lean, kg 8.5 6.1 8.1 7.6 8.4 6.2 9.2 2.6 P Fat-free lean, kg 73.3 60.3 60.5 69.0 61.1 64.0 78.0 10.1 P Total fatd, kg 28.4 23.4 25.6 29.8 26.3 31.9 31.2 6.8 Protein in lean, kg 11.3 8.3 10.4 10.3 7.6 10.3 11.1 2.6 P Change in LBW, kg −31.8 −24.0 −11.6 −24.9 −15.7 4.6 23.0 P, F −9.0 Change in lean, kg −15.2 −13.0 −5.0 −12.2 −10.8 2.6 11.0 P, F Change in fat-in-lean, kg −2.4 −0.4 −0.9 −0.1 −2.3 0.7 2.6
Change in fat-free lean, kg −13.0 −12.8 −4.3 −12.2 −9.3 4.7 10.1 P
Change in total fat, kg −5.0 −2.8 1.4 −0.6 3.5 2.8 6.6
Change in protein in lean, kg −3.0 −0.9 −1.0 −3.7 −1.0 −0.2 2.6 P Change in loin eye area, cm2 −22.6 −23.4 −11.1 −22.6 −18.7 3.7 18.1 P
aHP=high protein, MP=medium protein, and LP=low protein. Low fat was 2% added fat; high fat was 7% added fat. bIS=initial slaughter data, 15 sows were killed between d 1 and 3 of lactation to provide this data.
cP=effect of protein, F=effect of fat, P×F=interaction of protein and fat, all atP<0.05 or lower. dTotal fat=sum of abdominal, trim, and lean fat.
high producing sows. Such statistical comparisons do not answer questions on cause and effect; however, they do point out biological processes that may be more or less important for further experimentation on control mechanisms. In addition, these relationships can help set parameters in empirical and mechanistic models much better than can simple treatment means. The amount of lean in the carcasses of sows over the entire data set was closely related to the body weight (Lean Weight=0.440×BW−14.78 kg; r2=0.84). The change in BW and change in lean during lactation were also fairly well correlated (Figure 1), as has been noted
pre-Figure 1. Relation of change in body trimmed lean with change in body weight in sows fed varying protein and energy and nursing large litters. Change in total trimmed lean related to change in total BW during lactation. Car-cass composition change was calculated by subtracting that at 20 d of lactation from that in a comparative slaugh-ter group at d 1 to 3 of lactation. The equation describing the linear fit is: Change in body lean, kg=0.4146×Change in BW, kg−1.278, r2=0.840, n=52, SE
yx= 4.86 kg.
viously (Pettigrew et al., 1992a; Tokach et al., 1992), suggesting that a large percentage of the change in BW during lactation is due to gain or loss of carcass protein and fat.
The relationship between body lean and loin eye area was (LEA (cm2) = 0.401 × Lean + 33.8 kg, r2= 0.40). Also, the loss of body lean and the reduction in loin eye area were related but only with a regression coefficient of 0.26 (Figure 2). This would only weakly support the idea that LEA measures may have utility for estimating loss of carcass lean during lactation. Nevertheless, this
Figure 2. Relation of change in body lean to change in loin eye area in sows fed varying protein and energy and nursing large litters. Change in area of loin eye related to change in mass of trimmed lean during lactation. Change was calculated by subtracting value at d 20 of lactation from the comparative slaughter average mea-sured from d 1 to 3 of lactation. Equation for linear fit of data: loin eye area, cm2 = −8.92 + 0.404 × change in trimmed lean, kg, r2=0.26, n=37, SE
technique is less costly, obviously less invasive, and may help in practical research studies designed to de-termine the effects of feeding various protein levels or to determine protein requirements for maintenance of sow muscle during lactation.
The amount of body fat (carcass plus trim) was re-lated to body weight in lactating sows (Body Fat=0.174
× BW × 1.48 kg, r2 = 0.35), but the strength of this regression demonstrates that there are several other contributors to changes in body weight during lactation than body fat. The amount of body fat and body weight loss were also correlated with a regression coefficient of 0.40 (Figure 3). Interpreting the relation of BW loss, body protein (and associated muscle water) loss, and body fat loss, one might infer from this study that loss of body protein was the major contributor to loss of body weight and loss of body fat a lesser, but significant, contributor. The loss of fat in the trimmed lean was correlated with the loss of lean at approximately 40% as well (Figure 4). This has been noted in cows previously (Komaragiri and Erdman, 1997). The point here is not whether or not each regression coefficient is close to 1.0 but the overall pattern of changes in body protein, fat, and weight loss within a range. Certainly one would expect that the intercept, slope, and regression coeffi-cient of this relationship would be a function of the range of treatments applied and the severity of the deficit (or excess) in energy and amino acids. That is, lactating animals, which are deficient in both fat and amino acids, would be more likely to lose both body lean and body fat than those in a deficit only of fat. So, one goal is to conduct sufficient trials over sufficient
Figure 3. Relation of change in total body fat with change in body weight in sows fed varying protein and energy and nursing large litters. Change in total fat (fat in lean and trimmed fat) related to change in BW during lactation. Change was calculated by subtracting value at d 20 of lactation from the comparative slaughter average measured from d 1 to 3 of lactation. Equation for linear fit of data: change in total fat, kg= 1.35+ 0.1466× BW, kg; r2=0.379, n=48, SE
yx=5.19 kg.
Figure 4. Relation of change in fat contained in trim lean with change in total trimmed lean in sows fed vary-ing protein and energy and nursvary-ing large litters. Change in fat in trimmed lean only related to change in trimmed lean during lactation. Change was calculated by sub-tracting value at d 20 of lactation from the comparative slaughter average measured from d 1 to 3 of lactation. Equation for linear fit of data: change in fat in trimmed lean, kg=0.340+ 0.1414×trimmed lean, kg; r2=0.395, n=34, SEyx=2.04 kg.
ranges to put together a metaanalysis of these biological processes. Also, in some previous studies with sows, no such relationship has been noted. In fact, feeding additional protein to sows resulted in an increase in nitrogen balance but a loss in backfat thickness (King et al., 1993; Tokach et al., 1992). Recently, feeding two widely different (8.3 and 20.6%) amounts of protein to sows nursing 13 pigs, Jones and Stahly (1999) reported greater (but not significantly) total body fat loss on the higher protein ration. In that study, the sows on the greater protein diet also consumed more feed and lost less total weight. The interpretation was that the in-crease in protein supply stimulated more milk produc-tion, resulting in a need for more fat.
However, a different result obtained in the present study, in that even at protein intakes greater than what was the requirement in 1988, decreasing protein intake while maintaining total feed and energy intake in-creased the treatment means for protein loss and fat loss (admittedly the greater loss of fat on the LP rations was not significantly different). This result was also supported by the empirical relationship shown in Fig-ure 3 and 4: the greater the loss in body weight or lean, the greater the loss of body fat or of fat in the lean. There was also on average (not significant;P>0.10) a loss of body fat on LP treatments and no change or gain on MP and HP treatments. The explanation for this relationship is certainly neither simple nor clear. One may be random chance, which can only be ruled out by further repetition. On a strictly anatomical basis, it may be that as muscle protein is broken down, fiber
Table 8. Composition of a mammary gland of sows fed varying amounts of proteinaand fat during lactationb
Low fatb High fat
Item IS LP MP HP LP MP HP SD
Total wet wt, g 708.4 681.4 782.1 755.8 883 792.2 877.1 192.5*
Skin, g 71.1 75 72.8 78.1 87.7 80.2 92.2 21.4
Muscle, g 24.3 18.8 31.8 9.3 34.8 29.3 42.1 23.5
Trimmed fat, g 162.1 169 172.9 159.8 191.4 168.4 159 68.3
Trimmed gland (parenchyma)cg 466 418.6 511.0 508.6 569.2 509.6 583.8 128.9* Sum parenchyma+trimmed fat, g 628.1 587.6 683.9 668.4 760.6 678 742.8 170.9 Lipid content of trimmed fatd, g 137.8 143.7 147.0 135.8 162.7 143.1 135.2 58.0
Lipid, % total wet tissue 19.5 21.1 18.8 18.0 18.4 18.1 15.4 5.0
Trimmed gland (parenchyma) composition
Dry matter, % trimmed gland 29.2 20.8 20.9 22.8 22.6 22.7 23.9 4.2
Dry matter, g 136.8 86.8 106.6 116.3 122.6 117.3 136.7 38.6
Protein, % dry parenchymal wt 40.0 58.1 55.3 55.3 52.2 54.6 54.9 8.4
Protein, g 53.7 50.2 59.0 63.6 62.9 57.1 76.4 15.8
Protein, % wet parenchyma wt 11.6 12.0 11.5 12.6 11.5 11.8 13.4 1.4 Sum dry parenchyma
plus lipid in trimmed fat, g 274.2 230.5 253.5 252.1 280.0 264.2 286.8 82.4 Dry parenchyma plus lipid,
% wet trimmed gland plus fat 43.1 38.3 36.8 37.8 38.0 38.5 37.9 5.2 Lipid, % of dry trimmed
parenchyma and fat 48.2 59.8 56.6 54.0 55.4 54.6 50.7 9.9
DNA content, mg/g wet wt 2.2 2.3 2.1 2.1 2.4 1.9 2.2 0.6
aHP=high protein, MP=medium protein, and LP=low protein. Low fat was 2% added fat; high fat was 7% added fat.
bThe fourth gland caudally from the first gland on the right half of the animal was taken for dissection. In the event that that gland was nonfunctional, the next functional caudal gland was taken.
cThis is the mammary parenchyma with the fat, muscle, and skin dissected away; however, no further dissection internally to this sample was done before homogenization and analysis.
dThis number is 85% of the trimmed fat (Pettigrew et al., 1992a).
*Fat effect (High>Low) atP<0.05 for total mammary wet wt and parenchyma.
shapes change and associated fat is also mobilized, and not necessarily in direct response to a deficit of energy or fat. Also, it may be that some of the fat lost from the intermuscular areas was recycled as fatty acids to the subcutaneous fat depots. Both lipogenesis and lipolysis in subcutaneous adipose tissue proceed at significant rates in lactating sows fed adequate energy (Parmley and McNamara, 1996). This is consistent with the lack of change in backfat thickness and little or no change in trimmed fat. Also, this study was conducted on sows producing a large amount of milk every day to nurse 11 to 12 pigs. Thus, some of the fat mobilized from the lean may have been used to support oxidative processes while sparing glucose and gluconeogenic amino acids. All of these speculations may only be tested by further experiments, which continue to be needed to improve our models of nutrient metabolism.
The total weight of a representative mammary was increased (P< 0.05) during lactation in sows fed diets with a greater fat content, but dietary protein content had no effect (P>0.10) on total mammary gland weight (Table 8). Of all the measurements taken, only for trimmed parenchyma was there an increase due to higher fat inclusion in the diet (P<0.05). There was a consistent (but not significant (P>0.10)) trend that the glands of sows fed the low protein, low-fat ration had the least mammary parenchyma and least total protein (Table 8), which is also consistent with this group of animals having the smallest starting body weights and
the smallest mammary glands overall in the carcass dissection (Table 6). Thus, any real treatment effect here is unlikely. These data are not in complete agreement with the few data sets that do exist on mam-mary composition, but the basic weight ranges are con-sistent. Dourmad et al. (1998) reported a 1 kg increase in udder weight in sows fed 15.5% CP and 0.66% lysine and changes of+0.6 to+0.8 kg for those fed more lysine or more CP. The present data agree with mammary growth in general but would suggest that lower protein intakes would limit mammary growth. More recently, Kim et al. (1999) measured an increase of about 200 g per gland in wet weight from 5 to 21 d of lactation in glands that were known to have been suckled. Our measurement of total trimmed mammary gland weights would have included nonsuckled glands, and we measured about 690 g of total weight increase. How-ever, for the measurements on a single representative, suckled gland we measured a range of 50 to 180 g of increase per gland on animals fed diets considered nu-tritionally adequate (LFHP; and all HF diets). The DNA content (mg/g) of the mammary gland was not changed during lactation or by dietary treatment (Table 8). Our mean estimates of DNA (on a percentage basis) were quite close to those of Kim et al. (1999): they reported an increase in DNA concentration in wet tissue from 5 to 21 d of lactation from 0.21 to 0.26 percentage units. On a mass basis, they did report an increase of about 30% in mg of DNA in dry tissue and almost 100% in
total suckled glands. We determined an increase in mg of DNA from 1,558 mg at IS to 2,119 mg in the HFLP treatment at d 20, or a 36% increase (calculated from total wet gland wt and mg/d DNA in Table 8), so the results are generally consistent given the differences in the trials.
Implications
The modeling approach has been used with success in swine research and production. Adequacy of a model toward its objective is limited by data available to set equation forms and parameter values. The present data were compiled to challenge the behavior of an existing model of metabolism. Findings corroborate that de-creases in protein or fat intake limit milk production and grossly alter body composition of sows nursing larger litters. Protein and fat intake interact such that increased energy can partially relieve the effects of de-creased protein intake on milk production. Recommen-dations in the recent Nutrient Requirements of Swine are close to what we found optimal in this study. Sows mobilize amino acids from muscle to support mammary growth and milk production, and this is limiting only under a severe deficit of amino acids. The major impacts of this study are explored in the next paper in which the metabolic model is challenged with this data set.
Literature Cited
AOAC. 1990. Official Methods of Analysis. 15th ed. Assoc. Off. Anal. Chem., Washington, DC.
Baldwin, R. L. 1995. Modeling Ruminant Digestion and Metabolism. Chapman & Hall; New York.
Black, J. L., R. G. Campbell, I. H. Williams, K. J. James, and G. T. Davies. 1986. Simulation of energy and amino acid utilization in the pig. Res. and Dev. Agric. 3:121–145.
Bradford, M. M. 1976. A rapid and sensitive method for the quantita-tion of microgram quantities of protein utilizing the principles of protein-dye binding. Anal. Biochem. 72:248–256.
Clowes, E. J., I. H. Williams, V. E. Baracos, J. R. Pluske, A. C. Cegielski, L. J. Zak, and F. X. Aherne. 1998. Feeding lactating primiparous sows to establish three divergent metabolic states: II. Effect on nitrogen partitioning and skeletal muscle composi-tion. J. Anim. Sci. 76:1154–1164.
Cooper, D. R., J. F. Patience, R. T. Zijlstra, and M. Rademacher. 2001a. Effect of energy and lysine intake in gestation on sow performance. J. Anim. Sci. 79:2367–2377.
Cooper, D. R., J. F. Patience, R. T. Zilstra, and M. Rademacher. 2001b. Effect of nutrient intake in lactation on sow performance: Determining the threonine requirement of the high-producing lactating sow. J. Anim. Sci. 79:2378–2387.
Dourmad, J. Y., J. Noblet, and M. Etienne. 1998. Effect of protein and lysine supply on performance, nitrogen balance, and body composition changes of sows during lactation. J. Anim. Sci. 76:542–550.
Folch, J., J. Lees, and G. S. H. Stanley. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226:497–509.
Hurley, W. L., H. Wang, J. M. Bryson, and D. B. Shennan. 2000. Lysine uptake by mammary gland tissue from lactating sows. J. Anim. Sci. 78:391–395.
Jones, D. B., and T. S. Stahly 1999. Impact of amino acid nutrition during lactation on body nutrient mobilization and milk nutrient output in primiparous sows. J. Anim. Sci. 77:1513–1522. Kensinger, R. S., R. J. Collier, F. W. Bazer, C. A. Ducsay, and H. N.
Becker. 1982. Nucleic acid, metabolic and histological changes in gilt mammary tissue during pregnancy and lactogenesis. J. Anim. Sci. 54:1297–1308.
Kim, S. W., D. H. Baker, and R. A. Easter. 2001a. Dynamic ideal protein and limiting amino acids for lactating sows: The impact of amino acid mobilization. J. Anim. Sci. 79:2356–2366. Kim, S. W., R. A. Easter, and W. L. Hurley. 2001b. The regression
of unsuckled mammary glands during lactation in sows: The influence of lactation stage, dietary nutrients, and litter size. J. Anim. Sci. 79:2659–2668.
Kim, S. W., W. L. Hurley, I. K. Han, and R. A. Easter. 1999. Changes in tissue composition associated with mammary gland growth during lactation in sows. J. Anim. Sci. 77:2510–2516.
King, R. H., M. S. Toner, H. Dove, C. S. Atwood, and W. G. Brown. 1993. The response of first-litter sows to dietary protein level during lactation. J. Anim. Sci. 71:2457–2463.
Komaragiri, M. V. S., and R. A. Erdman. 1997. Factors affecting body tissue mobilization in early lactation dairy cows. 1. Effect of dietary protein on mobilization of body fat and protein. J. Dairy Sci. 80:929–937.
McNamara, J. P. 1998. Interaction of Glucose and amino acid metabo-lism in lactating sows: Estimating internal parameters of a model of metabolism. In: K. McKracken, E. F. Unsworth, and A. R. G. Wylie (ed.) Energy Metabolism of Farm Animals. pp 32–35. CAB International, London.
McNamara, J. P., and D. E. Boyd. 1998. Hormones as Quantitative Controllers. In: A Quantitative Biology of the Pig. pp 199–225. CAB International, London. NRC. 1988. Nutrient Requirements of Swine. 9th ed. National Academy Press, Washington, DC. NRC. 1998. Nutrient Requirements of Swine. 10th ed. National
Acad-emy Press, Washington, DC.
Noblet, J., and M. Etienne. 1989. Estimation of sow milk nutrient output. J. Anim. Sci. 67:3352–3359.
Parmley, K. L. S., and J. P. McNamara. 1996. Lipid Metabolism in Adipose Tissue of Pigs Fed Varying Amounts of Energy. J. Nutr. 126:1644–1656.
Pettigrew J. E., M. Gill, J. France, and W. H. Close. 1992a. A mathe-matical integration of energy and amino acid metabolism in lactating sows. J. Anim. Sci. 70:3742–3761.
Pettigrew J. E., M. Gill, J. France, and W. H. Close. 1992b. Evaluation of a mathematical model of lactating sow metabolism. J. Anim. Sci. 70:3762–3773.
Pluske, J. R., I. H. Williams, L. J. Zak, E. J. Clowes, A. C. Cegielski, and F. X. Aherne. 1998. Feeding lactating primiparous sows to establish three divergent metabolic states: III. Milk production and pig growth. J. Anim. Sci. 76:1165–1171.
Renaudeau, D., and J. Noblet. 2001. Effects of exposure to high ambi-ent temperature and dietary protein level on sow milk production and performance of piglets. J. Anim. Sci. 79:1540–1548. Steel, R. G. D., and J. H. Torrie. 1980. Principles and Procedures of
Statistics: A Biomedical Approach. 2nd ed. McGraw-Hill, New York.
Tokach, M. D., J. E. Pettigrew, B. A. Crooker, G. D. Dial, and A. F. Sower. 1992. Quantitative influence of lysine and energy intake on yield of milk components in the primiparous sow. J. Anim. Sci. 70:1864–1872.
Trottier, N. L., C. F. Shipley, and R. A. Easter. 1997. Plasma amino acid uptake by the mammary gland of the lactating sow. J. Anim. Sci. 75:1266–1278.
Yang, H., J. E. Pettigrew, L. J. Johnston, G. C. Shurson, J. E. Wheaton, M. E. White, Y. Koketsu, A. F. Sower, and J. A. Rath-macher. 2000. Effects of dietary lysine intake during lactation on blood metabolites, hormones, and reproductive performance in primiparous sows. J. Anim. Sci. 78:1001–1009.