inorganic papers
i64
Malin MaÊrtenssonet al. Ba2.125PdO0.422 DOI: 10.1107/S1600536801010935 Acta Cryst.(2001). E57, i64±i66 Acta Crystallographica Section EStructure Reports
Online ISSN 1600-5368
Ba
68Pd
32O
x, with
x
'
13.5
Malin MaÊrtensson, Emma Wikstad, Helen Blomqvist, Mikael Kritikos and Lars Eriksson*
Division of Structural Chemistry, Arrhenius Laboratory, Stockholm University, S-106 91 Stockholm, Sweden
Correspondence e-mail: lerik@struc.su.se
Key indicators Single-crystal X-ray study
T= 293 K
Mean(Ba±O) = 0.003 AÊ Disorder in solvent or counterion
Rfactor = 0.029
wRfactor = 0.041
Data-to-parameter ratio = 15.3
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2001 International Union of Crystallography Printed in Great Britain ± all rights reserved
The title compound, barium palladium oxide, Ba68Pd32Ox,x'
13.5 is a medium-sized cubic suboxide. Two of the four crystallographically independent Ba atoms form a network of face-sharing octahedra with O atoms occupying the central position of these octahedra, giving a network resembling the pyrochlore structure. Two of three O-atom positions are fully occupied and the third partially occupied. The remainder of the Ba atoms and the Pd atoms are distributed in another network residing in the channels of the network of face-sharing O±Ba6octahedra.
Comment
The title compound was synthesized as part of a search for intermetallic compounds for use as hydrogen storage mate-rials. The compound is composed of a network of face-sharing O±Ba6octahedra with the residual Ba atoms and all Pd atoms
located in the tunnels of the octahedra network. O atoms were assigned to model quite large residual densities in the centre of the octahedra. This network of O±Ba6is closely related to
the pyrochlore structure (Gaertner, 1930). Removing the O1 atom (Fig. 1) gives a good resemblance between the
pyro-chlore Nb±O6 octahedra and the network of O2±Ba26
octa-hedra in the title compound. Barium suboxides, with similar arrangements of O±Ba octahedra, have been observed earlier (RoÈhr, 1995). The main difference in the present compound are the slightly longer BaÐO and BaÐBa distances. This may be due to partial occupation of the O-atom positions giving a weaker attraction but may also be an effect of the excess Ba and Pd in the structure. In addition to the network of O±Ba6
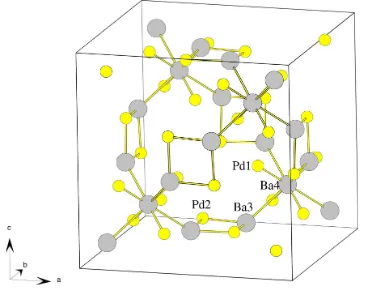
octahedra as shown in Fig. 2, one can construct a comple-mentary network of tetrahedraly coordinated Ba3 around Ba4 with an extra tetrahedron of Pd1 around Ba4 connected to
each other through squares of 2 Ba3 and 2 Pd2. This
additional network is shown in Fig. 3. It must be emphasized that the only indication that the title compound contains oxygen is the much better ®t of the diffraction data when the model includes O atoms. Removing the O atoms from the structure model gives a pure intermetallic compound with the composition Ba68Pd32. This is, however, probably not correct.
The oxygen stoichiometry cannot be stated with particularly high accuracy as it is a result from re®nement.
Experimental
The compound was crystallized from a solid-state reaction between a mixture of BaH2(Ba rods 99.9+%, Aldrich Chemical Company Inc.;
heated under 50 bar of H2pressure at 723 K for 4 h) and Pd (powder
< 60mm, claimed purity 99.9+%, Chempur) in a nominal molar ratio Ba:Pd = 2:1 mixed and heated (T'973 K) in an Al2O3crucible in a
stainless-steel reactor under a H2pressure of 35 bar. All materials
were handled in an argon-®lled glove-box. The reason for using
hydrogen was that the initial intention was to synthesize hydrides. The most probable sources of oxygen are either residual impurities in the glove-box atmosphere or a solid-state reaction with the crucible material (Al2O3). Assuming the conditions of Ellingham diagrams
(Wulfsberg, 1987) to be true, one can imagine that the reduction of Al2O3 with Ba metal would be spontaneous at the synthesis
temperature, thus a possible source of oxygen is the crucible material. Small single crystals were obtained from the solidi®ed reaction product.
Crystal data
Ba2.125PdO0.422
Mr= 404.91
Cubic,F43m a= 15.878 (1) AÊ V= 4003.0 (4) AÊ3
Z= 32
Dx= 5.340 Mg mÿ3
MoKradiation
Cell parameters from 32 re¯ections
= 15.0±19.2
= 19.89 mmÿ1
T= 293 (2) K
Prism, metallic light grey 0.140.090.07 mm
Data collection
Stoe AED-4 diffractometer
!/2scans
Absorption correction: numerical (X-RED; Stoe & Cie, 1997) Tmin= 0.060,Tmax= 0.259
1671 measured re¯ections 336 independent re¯ections 270 re¯ections withI> 2(I)
Rint= 0.079
max= 27.9
h=ÿ1!20
k=ÿ1!20
l=ÿ1!20
3 standard re¯ections frequency: 240 min intensity decay: 1%
Re®nement
Re®nement onF2
R[F2> 2(F2)] = 0.029
wR(F2) = 0.042
S= 1.16 336 re¯ections 22 parameters w= 1/[2(F
o2) + (0.01P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 1.65 e AÊÿ3
min=ÿ1.32 e AÊÿ3
Absolute structure: Flack (1983), 45 Friedel pairs
Flack parameter =ÿ0.08 (11) Figure 2
Slightly more than the unit cell content with the network of O±Ba6
octahedra shown blue around O1, green around O2 and red around O3. Pd atoms are yellow and Ba grey. The Ba atoms that form the octahedra
Figure 3
The unit-cell content with the complementary network to the O±Ba6
network. The Ba atoms are shown as grey circles and Pd with yellow circles. The orientation of this picture is equivalent to Fig. 2.
Figure 1
Part of the network of O±Ba6octahedra. The octahedra with O1 in its
inorganic papers
i66
Malin MaÊrtenssonet al. Ba2.125PdO0.422 Acta Cryst.(2001). E57, i64±i66Table 1
Selected distances (AÊ).
Ba1ÐO1i 2.7999 (16)
Ba1ÐO3 3.00 (4) Ba1ÐPd2ii 3.255 (2)
Ba1ÐBa1iii 3.960 (2)
Ba1ÐBa2iv 4.3277 (6)
Ba2ÐO2i 2.7029 (13)
Ba2ÐO3v 2.82 (3)
Ba2ÐPd1vi 3.280 (2)
Ba2ÐBa2vii 3.8225 (18)
Ba2ÐBa3 4.2849 (11) Ba3ÐPd2viii 3.2825 (17)
Ba3ÐBa4 4.1393 (18) Ba3ÐBa1ix 4.3567 (11)
Ba3ÐBa3x 4.468 (3)
Ba4ÐPd1 2.888 (3)
Symmetry codes: (i) x;yÿ1
2;zÿ12; (ii) x;1ÿy;1ÿz; (iii) 12z;1ÿx;12ÿy; (iv)
1ÿy;1
2z;12ÿx; (v) xÿ12;12ÿy;ÿz; (vi) x;12ÿy;12ÿz; (vii) 12ÿy;z;12ÿx; (viii)
xÿ1;1ÿy;1ÿz; (ix)y;z;xÿ1; (x)x;ÿy;ÿz.
The O atoms in the centre of the barium octahedra were located from difference-density maps. Without the O atoms, residual densi-ties of 12.2, 7.4 and 2.9 e AÊÿ3were located in the centre of the Ba
octahedra. Adding the O atoms further decreased the conventional R1 from 0.040 to 0.029. All O atoms were re®ned with a common isotropic displacement parameter and the occupancy of O3 was left free to re®ne. Adding hydrogen as central atoms to describe the residual intensities invariably led to negative displacement para-meters and the only reasonable model that ®tted the diffraction data was the one described with the O atoms in the centre of the octa-hedra. The corresponding re®nement of the occupancy parameters of O1 and O2 did not yield any signi®cant deviation from full occupancy, thus they were ®xed at full occupancy. The use of a common isotropic
displacement parameter was found to be the model that gave the most stable re®nement. No chemical analysis of the O content was performed; thus the only indication of the presence of O atoms is the better ®t to the structural data.
Data collection:DIF4 (Stoe & Cie, 1988); cell re®nement:DIF4; data reduction:REDU4 (Stoe & Cie, 1988); program(s) used to solve structure: SHELXS97 (Sheldrick, 1990); program(s) used to re®ne structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
DIAMOND(Bergerhoff, 1996).
References
Bergerhoff, G. (1996).DIAMOND. Gerhard-Domagk-Straûe 1, 53121 Bonn, Germany.
Flack, H. D. (1983).Acta Cryst.A39, 876±881.
Gaertner, H. R. von (1930). Zentralblatt fuer Mineralogie, Geologie und Paleontologie, Abt. A., pp. 1±30. Stuttgart: E. Schweizerbart'sche Verlags-buchhandlung.
RoÈhr, C. (1995).Z. Anorg. Allg. Chem.621, 1496±1500. Sheldrick, G. M. (1990).Acta Cryst.A46, 467±473.
Sheldrick, G. M. (1997).SHELXL97. University of GoÈttingen, Germany. Stoe & Cie (1988).AED-2,DIF4 (Version 7.04) andREDU4 (Version 6.2).
Stoe & Cie GmbH, Darmstadt, Germany.
Stoe & Cie (1997).X-RED. Version 1.09. Stoe & Cie GmbH, Darmstadt, Germany.
supporting information
Acta Cryst. (2001). E57, i64–i66 [doi:10.1107/S1600536801010935]
Ba
68Pd
32O
x, with
x
[\simeq] 13.5
Malin M
å
rtensson, Emma Wikstad, Helen Blomqvist, Mikael Kritikos and Lars Eriksson
S1. Comment
The title compound was synthesized as part of a search for intermetallic compounds for use as hydrogen storage
materials. The compound is composed of a network of face sharing O–Ba6 octahedra with the residual Ba atoms and all
Pd atoms located in the tunnels of the octahedra network. O atoms were assigned to model quite large residual densities
in the centre of the octahedra. This network of O—Ba6 is closely related to the pyrochlore structure (Gaertner, 1930).
Removing the O1 atom (Fig. 1) gives a good resemblance between the pyrochlore Nb—O6 octahedra and the network of
O2—Ba26 octahedra in the title compound. Barium suboxides, with similar arrangements of O—Ba octahedra, have been
observed earlier (Röhr, 1995). The main difference in the present compound are the slightly longer Ba—O and Ba—Ba
distances. This may be due to partial occupation of the O-atom positions giving a weaker attraction but may also be an
effect of the excess Ba and Pd in the structure. In addition to the network of O—Ba6 octahedra as shown in Fig. 2, one
can construct a complementary network of tetrahedraly coordinated Ba3 around Ba4 with an extra tetrahedron of Pd1
around Ba4 connected to each other through squares of 2 × Ba3 and 2 × Pd2. This additional network is shown in Fig. 3.
It must be emphasized that the only indication that the title compound contains oxygen is the much better fit of the
diffraction data when the model includes O atoms. Removing the O atoms from the structure model gives a pure
intermetallic compound with the composition Ba68Pd32. This is, however, probably not correct. The oxygen stoichiometry
cannot be stated with particularly high accuracy as it is a result from refinement.
S2. Experimental
The compound was crystallized from a solid-state reaction between a mixture of BaH2 (Ba rods 99.9+%, Aldrich
Chemical Company Inc.; heated under 50 bar of H2 pressure at 723 K for 4 h) and Pd (powder < 60 µm, claimed purity
99.9+%, Chempur) in a nominal molar ratio Ba:Pd = 2:1 mixed and heated (T ≈ 973 K) in an Al2O3 crucible in a stainless-steel reactor under a H2 pressure of 35 bar. All materials were handled in an argon-filled glove-box. The reason
for using hydrogen was that the initial intention was to synthesize hydrides. The most probable sources of oxygen are
either residual impurities in the glove-box atmosphere or a solid-state reaction with the crucible material (Al2O3).
Assuming the conditions of Ellingham diagrams (Wulfsberg, 1987) to be true, one can imagine that the reduction of Al2O3
with Ba metal would be spontaneous at the synthesis temperature, thus a possible source of oxygen is the crucible
material. Small single crystals were obtained from the solidified reaction product.
S3. Refinement
The O atoms in the center of barium octahedra were located from difference-density maps. Without the O atoms, residual
densities of 12.2, 7.4 and 2.9 e Å-3 were located in the center of the Ba octahedra. Adding the O atoms further decreased
the conventional R1 from 0.040 to 0.029. A l l O atoms were refined with a common isotropic displacement parameter
supporting information
sup-2
Acta Cryst. (2001). E57, i64–i66invariably led to negative displacement parameters and the only reasonable model that fitted the diffraction data was the
one described with the oxygen atoms in the center of the octahedra. The corresponding refinement of the occupancy
parameters of O1 and O2 did not yield any significant deviation from full occupancy, thus they were locked at full
occupancy. The use of a common isotropic displacement parameter was found to be the model that gave the most stable
refinement. No chemical analysis of the O content were done, thus the only indication of the presence of O atoms is the
[image:5.610.129.486.167.531.2]better fit to the structural data.
Figure 1
Part of the network of O—Ba6 octahedra. The octahedra with O1 in its centre is blue and the ones with O3 in the centres
are red. The Pd atoms are shown as yellow unconnected circles and the Ba atoms are shown with small grey circles at the
Figure 2
Slightly more than the unit-cell content with the network of O—Ba6 octahedra shown blue around O1, green around O2
supporting information
[image:7.610.123.488.72.365.2]sup-4
Acta Cryst. (2001). E57, i64–i66Figure 3
The unit-cell content with the complementary network to the O–Ba6 network. The Ba atoms are shown as grey circles and
Pd with yellow circles. The orientation of picture is equivalent to Fig. 2.
barium palladium oxide
Crystal data
Ba2.125PdO0.422
Mr = 404.91 Cubic, F43m a = 15.878 (1) Å
V = 4003.0 (4) Å3
Z = 32
F(000) = 5344
Dx = 5.340 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 32 reflections
θ = 15.0–19.2°
µ = 19.89 mm−1
T = 293 K
Prism, metallic light grey 0.14 × 0.09 × 0.07 mm
Data collection
Stoe AED4 diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω/2θ scans
Absorption correction: numerical (X-RED; Stoe & Cie, 1997)
Tmin = 0.060, Tmax = 0.259 1671 measured reflections
336 independent reflections 270 reflections with I > 2σ(I)
Rint = 0.079
θmax = 27.9°, θmin = 2.2°
h = −1→20
k = −1→20
l = −1→20
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.029
wR(F2) = 0.042
S = 1.16 336 reflections 22 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
w = 1/[σ2(F
o2) + (0.01P)2] where P = (Fo2 + 2Fc2)/3 (Δ/σ)max < 0.001
Δρmax = 1.65 e Å−3 Δρmin = −1.32 e Å−3
Absolute structure: Flack (1983), XXXX Friedel pairs
Absolute structure parameter: −0.08 (11)
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq Occ. (<1)
Ba1 0.92634 (10) 0.2500 0.2500 0.0160 (5)
Ba2 0.32977 (8) 0.0000 0.0000 0.0153 (5)
Ba3 0.09949 (6) 0.09949 (6) 0.09949 (6) 0.0128 (4)
Ba4 0.2500 0.2500 0.2500 0.0119 (7)
Pd1 0.35502 (11) 0.35502 (11) 0.35502 (11) 0.0140 (5) Pd2 0.89303 (10) 0.89303 (10) 0.89303 (10) 0.0114 (6)
O1 0.7500 0.7500 0.7500 0.011 (5)*
O2 0.5000 0.5000 0.5000 0.011 (5)*
O3 0.879 (2) 0.379 (2) 0.121 (2) 0.011 (5)* 0.34 (3)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Ba1 0.0113 (9) 0.0184 (6) 0.0184 (6) 0.000 0.000 −0.0105 (7)
Ba2 0.0087 (8) 0.0187 (5) 0.0187 (5) 0.000 0.000 0.0000 (10)
Ba3 0.0128 (4) 0.0128 (4) 0.0128 (4) 0.0015 (5) 0.0015 (5) 0.0015 (5)
Ba4 0.0119 (7) 0.0119 (7) 0.0119 (7) 0.000 0.000 0.000
Pd1 0.0140 (5) 0.0140 (5) 0.0140 (5) −0.0032 (7) −0.0032 (7) −0.0032 (7) Pd2 0.0114 (6) 0.0114 (6) 0.0114 (6) −0.0014 (7) −0.0014 (7) −0.0014 (7)
Geometric parameters (Å, º)
Ba1—O1i 2.7999 (16) Ba3—Ba3xxv 4.468 (3)
Ba1—O3 3.00 (4) Ba4—Pd1 2.888 (3)
supporting information
sup-6
Acta Cryst. (2001). E57, i64–i66Ba1—Pd2iii 3.255 (2) Ba4—Pd1xxvi 2.888 (3)
Ba1—Pd2i 3.255 (2) Ba4—Pd1xxvii 2.888 (3)
Ba1—Ba1iv 3.960 (2) Ba4—Ba3xxvii 4.1393 (18)
Ba1—Ba1v 3.960 (2) Ba4—Ba3xxvi 4.1393 (18)
Ba1—Ba1vi 3.960 (2) Ba4—Ba3ii 4.1393 (18)
Ba1—Ba1vii 3.960 (2) Pd1—Ba2xxviii 3.280 (2)
Ba1—Ba2viii 4.3277 (6) Pd1—Ba2xxix 3.280 (2)
Ba1—Ba2ix 4.3277 (6) Pd1—Ba2xxx 3.280 (2)
Ba1—Ba2x 4.3277 (6) Pd2—Ba1xxviii 3.255 (2)
Ba2—O2i 2.7029 (13) Pd2—Ba1xxx 3.255 (2)
Ba2—O3xi 2.82 (3) Pd2—Ba1xxix 3.255 (2)
Ba2—O3xii 2.82 (3) Pd2—Ba3xxxi 3.2825 (17)
Ba2—Pd1ii 3.280 (2) Pd2—Ba3xxxii 3.2825 (17)
Ba2—Pd1i 3.280 (2) Pd2—Ba3xxxiii 3.2825 (17)
Ba2—Ba2xiii 3.8225 (18) O1—Ba1xxxiv 2.7999 (16)
Ba2—Ba2xiv 3.8225 (18) O1—Ba1xxx 2.7999 (16)
Ba2—Ba2vi 3.8225 (18) O1—Ba1xxviii 2.7999 (16)
Ba2—Ba2vii 3.8225 (18) O1—Ba1xxix 2.7999 (16)
Ba2—Ba3xv 4.2849 (11) O1—Ba1xxxv 2.7999 (16)
Ba2—Ba3 4.2849 (11) O1—Ba1xxxvi 2.7999 (16)
Ba2—Ba1xvi 4.3277 (6) O2—Ba2xxxvii 2.7029 (13)
Ba3—Pd2xvii 3.2825 (17) O2—Ba2xxviii 2.7029 (13)
Ba3—Pd2xviii 3.2825 (17) O2—Ba2xxxviii 2.7029 (13)
Ba3—Pd2xix 3.2825 (17) O2—Ba2xxix 2.7029 (13)
Ba3—Ba4 4.1393 (18) O2—Ba2iv 2.7029 (13)
Ba3—Ba2xx 4.2849 (11) O2—Ba2xxx 2.7029 (13)
Ba3—Ba2xxi 4.2849 (11) O3—Ba2viii 2.82 (3)
Ba3—Ba1xxii 4.3567 (11) O3—Ba2xxxix 2.82 (3)
Ba3—Ba1xxiii 4.3567 (11) O3—Ba2x 2.82 (3)
Ba3—Ba1xxiv 4.3567 (11) O3—Ba1v 3.00 (4)
Ba3—Ba3xv 4.468 (3) O3—Ba1vi 3.00 (4)
O1i—Ba1—O3 75.6 (8) Ba2—Ba3—Ba2xx 119.556 (6)
O1i—Ba1—O3ii 75.6 (8) Pd2xvii—Ba3—Ba2xxi 67.311 (13)
O3—Ba1—O3ii 151.3 (16) Pd2xviii—Ba3—Ba2xxi 151.51 (7)
O1i—Ba1—Pd2iii 80.65 (4) Pd2xix—Ba3—Ba2xxi 67.311 (13)
O3—Ba1—Pd2iii 87.69 (13) Ba4—Ba3—Ba2xxi 86.16 (3)
O3ii—Ba1—Pd2iii 87.69 (13) Ba2—Ba3—Ba2xxi 119.556 (6)
O1i—Ba1—Pd2i 80.65 (4) Ba2xx—Ba3—Ba2xxi 119.556 (6)
O3—Ba1—Pd2i 87.69 (13) Pd2xvii—Ba3—Ba1xxii 123.422 (14)
O3ii—Ba1—Pd2i 87.69 (13) Pd2xviii—Ba3—Ba1xxii 123.422 (14) Pd2iii—Ba1—Pd2i 161.30 (9) Pd2xix—Ba3—Ba1xxii 47.94 (4)
O1i—Ba1—Ba1iv 45.0 Ba4—Ba3—Ba1xxii 74.39 (3)
O3—Ba1—Ba1iv 108.0 (7) Ba2—Ba3—Ba1xxii 60.099 (5)
O3ii—Ba1—Ba1iv 48.7 (6) Ba2xx—Ba3—Ba1xxii 160.55 (5)
Pd2iii—Ba1—Ba1iv 52.54 (3) Ba2xxi—Ba3—Ba1xxii 60.099 (5) Pd2i—Ba1—Ba1iv 112.24 (4) Pd2xvii—Ba3—Ba1xxiii 123.422 (14)
O3—Ba1—Ba1v 48.7 (6) Pd2xix—Ba3—Ba1xxiii 123.422 (14)
O3ii—Ba1—Ba1v 108.0 (7) Ba4—Ba3—Ba1xxiii 74.39 (3)
Pd2iii—Ba1—Ba1v 52.54 (3) Ba2—Ba3—Ba1xxiii 60.099 (5)
Pd2i—Ba1—Ba1v 112.24 (4) Ba2xx—Ba3—Ba1xxiii 60.099 (5)
Ba1iv—Ba1—Ba1v 60.0 Ba2xxi—Ba3—Ba1xxiii 160.55 (5)
O1i—Ba1—Ba1vi 45.0 Ba1xxii—Ba3—Ba1xxiii 113.04 (2)
O3—Ba1—Ba1vi 48.7 (6) Pd2xvii—Ba3—Ba1xxiv 47.94 (4)
O3ii—Ba1—Ba1vi 108.0 (7) Pd2xviii—Ba3—Ba1xxiv 123.422 (14) Pd2iii—Ba1—Ba1vi 112.24 (4) Pd2xix—Ba3—Ba1xxiv 123.422 (14)
Pd2i—Ba1—Ba1vi 52.54 (3) Ba4—Ba3—Ba1xxiv 74.39 (3)
Ba1iv—Ba1—Ba1vi 90.0 Ba2—Ba3—Ba1xxiv 160.55 (5)
Ba1v—Ba1—Ba1vi 60.0 Ba2xx—Ba3—Ba1xxiv 60.099 (5)
O1i—Ba1—Ba1vii 45.0 Ba2xxi—Ba3—Ba1xxiv 60.099 (5)
O3—Ba1—Ba1vii 108.0 (7) Ba1xxii—Ba3—Ba1xxiv 113.04 (2)
O3ii—Ba1—Ba1vii 48.7 (6) Ba1xxiii—Ba3—Ba1xxiv 113.04 (2) Pd2iii—Ba1—Ba1vii 112.24 (4) Pd2xvii—Ba3—Ba3xv 92.93 (5) Pd2i—Ba1—Ba1vii 52.54 (3) Pd2xviii—Ba3—Ba3xv 47.11 (4)
Ba1iv—Ba1—Ba1vii 60.0 Pd2xix—Ba3—Ba3xv 47.11 (4)
Ba1v—Ba1—Ba1vii 90.0 Ba4—Ba3—Ba3xv 144.7
Ba1vi—Ba1—Ba1vii 60.0 Ba2—Ba3—Ba3xv 58.58 (3)
O1i—Ba1—Ba2viii 105.68 (2) Ba2xx—Ba3—Ba3xv 110.04 (2)
O3—Ba1—Ba2viii 40.4 (6) Ba2xxi—Ba3—Ba3xv 110.04 (2)
O3ii—Ba1—Ba2viii 153.62 (9) Ba1xxii—Ba3—Ba3xv 86.66 (2) Pd2iii—Ba1—Ba2viii 118.664 (16) Ba1xxiii—Ba3—Ba3xv 86.66 (2) Pd2i—Ba1—Ba2viii 66.93 (2) Ba1xxiv—Ba3—Ba3xv 140.87 (3) Ba1iv—Ba1—Ba2viii 147.11 (2) Pd2xvii—Ba3—Ba3xxv 47.11 (4) Ba1v—Ba1—Ba2viii 89.09 (2) Pd2xviii—Ba3—Ba3xxv 47.11 (4) Ba1vi—Ba1—Ba2viii 62.776 (19) Pd2xix—Ba3—Ba3xxv 92.93 (5)
Ba1vii—Ba1—Ba2viii 113.456 (15) Ba4—Ba3—Ba3xxv 144.7
O1i—Ba1—Ba2ix 105.68 (2) Ba2—Ba3—Ba3xxv 110.04 (2)
O3—Ba1—Ba2ix 153.62 (9) Ba2xx—Ba3—Ba3xxv 58.58 (3)
O3ii—Ba1—Ba2ix 40.4 (6) Ba2xxi—Ba3—Ba3xxv 110.04 (2)
Pd2iii—Ba1—Ba2ix 118.664 (16) Ba1xxii—Ba3—Ba3xxv 140.87 (3) Pd2i—Ba1—Ba2ix 66.93 (2) Ba1xxiii—Ba3—Ba3xxv 86.66 (2) Ba1iv—Ba1—Ba2ix 89.09 (2) Ba1xxiv—Ba3—Ba3xxv 86.66 (2)
Ba1v—Ba1—Ba2ix 147.11 (2) Ba3xv—Ba3—Ba3xxv 60.0
Ba1vi—Ba1—Ba2ix 113.456 (15) Pd1—Ba4—Pd1ii 109.5
Ba1vii—Ba1—Ba2ix 62.776 (19) Pd1—Ba4—Pd1xxvi 109.5
Ba2viii—Ba1—Ba2ix 117.64 (4) Pd1ii—Ba4—Pd1xxvi 109.5
O1i—Ba1—Ba2x 105.68 (2) Pd1—Ba4—Pd1xxvii 109.5
O3—Ba1—Ba2x 40.4 (6) Pd1ii—Ba4—Pd1xxvii 109.5
O3ii—Ba1—Ba2x 153.62 (9) Pd1xxvi—Ba4—Pd1xxvii 109.5
Pd2iii—Ba1—Ba2x 66.93 (2) Pd1—Ba4—Ba3 180.00 (3)
Pd2i—Ba1—Ba2x 118.664 (16) Pd1ii—Ba4—Ba3 70.5
Ba1iv—Ba1—Ba2x 113.456 (15) Pd1xxvi—Ba4—Ba3 70.5
Ba1v—Ba1—Ba2x 62.776 (19) Pd1xxvii—Ba4—Ba3 70.5
Ba1vi—Ba1—Ba2x 89.09 (2) Pd1—Ba4—Ba3xxvii 70.5
supporting information
sup-8
Acta Cryst. (2001). E57, i64–i66Ba2viii—Ba1—Ba2x 52.42 (3) Pd1xxvi—Ba4—Ba3xxvii 70.5 Ba2ix—Ba1—Ba2x 148.64 (4) Pd1xxvii—Ba4—Ba3xxvii 180.00 (3)
O2i—Ba2—O3xi 73.8 (9) Ba3—Ba4—Ba3xxvii 109.5
O2i—Ba2—O3xii 73.8 (9) Pd1—Ba4—Ba3xxvi 70.5
O3xi—Ba2—O3xii 147.5 (18) Pd1ii—Ba4—Ba3xxvi 70.5
O2i—Ba2—Pd1ii 82.98 (4) Pd1xxvi—Ba4—Ba3xxvi 180.00 (3)
O3xi—Ba2—Pd1ii 88.04 (10) Pd1xxvii—Ba4—Ba3xxvi 70.5
O3xii—Ba2—Pd1ii 88.04 (10) Ba3—Ba4—Ba3xxvi 109.5
O2i—Ba2—Pd1i 82.98 (4) Ba3xxvii—Ba4—Ba3xxvi 109.5
O3xi—Ba2—Pd1i 88.04 (10) Pd1—Ba4—Ba3ii 70.5
O3xii—Ba2—Pd1i 88.04 (10) Pd1ii—Ba4—Ba3ii 180.00 (8)
Pd1ii—Ba2—Pd1i 165.96 (9) Pd1xxvi—Ba4—Ba3ii 70.5
O2i—Ba2—Ba2xiii 45.0 Pd1xxvii—Ba4—Ba3ii 70.5
O3xi—Ba2—Ba2xiii 106.4 (7) Ba3—Ba4—Ba3ii 109.5
O3xii—Ba2—Ba2xiii 47.3 (6) Ba3xxvii—Ba4—Ba3ii 109.5
Pd1ii—Ba2—Ba2xiii 54.36 (4) Ba3xxvi—Ba4—Ba3ii 109.5
Pd1i—Ba2—Ba2xiii 114.20 (4) Ba4—Pd1—Ba2xxviii 137.72 (4)
O2i—Ba2—Ba2xiv 45.0 Ba4—Pd1—Ba2xxix 137.72 (4)
O3xi—Ba2—Ba2xiv 47.3 (6) Ba2xxviii—Pd1—Ba2xxix 71.28 (7)
O3xii—Ba2—Ba2xiv 106.4 (7) Ba4—Pd1—Ba2xxx 137.72 (4)
Pd1ii—Ba2—Ba2xiv 54.36 (4) Ba2xxviii—Pd1—Ba2xxx 71.28 (7) Pd1i—Ba2—Ba2xiv 114.20 (4) Ba2xxix—Pd1—Ba2xxx 71.28 (7) Ba2xiii—Ba2—Ba2xiv 60.0 Ba1xxviii—Pd2—Ba1xxx 74.93 (7)
O2i—Ba2—Ba2vi 45.0 Ba1xxviii—Pd2—Ba1xxix 74.93 (7)
O3xi—Ba2—Ba2vi 106.4 (7) Ba1xxx—Pd2—Ba1xxix 74.93 (7)
O3xii—Ba2—Ba2vi 47.3 (6) Ba1xxviii—Pd2—Ba3xxxi 135.74 (2) Pd1ii—Ba2—Ba2vi 114.20 (4) Ba1xxx—Pd2—Ba3xxxi 83.58 (4) Pd1i—Ba2—Ba2vi 54.36 (4) Ba1xxix—Pd2—Ba3xxxi 135.74 (2) Ba2xiii—Ba2—Ba2vi 60.0 Ba1xxviii—Pd2—Ba3xxxii 83.58 (4)
Ba2xiv—Ba2—Ba2vi 90.0 Ba1xxx—Pd2—Ba3xxxii 135.74 (2)
O2i—Ba2—Ba2vii 45.0 Ba1xxix—Pd2—Ba3xxxii 135.74 (2)
O3xi—Ba2—Ba2vii 47.3 (6) Ba3xxxi—Pd2—Ba3xxxii 85.78 (8) O3xii—Ba2—Ba2vii 106.4 (7) Ba1xxviii—Pd2—Ba3xxxiii 135.74 (2) Pd1ii—Ba2—Ba2vii 114.20 (4) Ba1xxx—Pd2—Ba3xxxiii 135.74 (2) Pd1i—Ba2—Ba2vii 54.36 (4) Ba1xxix—Pd2—Ba3xxxiii 83.58 (4) Ba2xiii—Ba2—Ba2vii 90.0 Ba3xxxi—Pd2—Ba3xxxiii 85.78 (8) Ba2xiv—Ba2—Ba2vii 60.0 Ba3xxxii—Pd2—Ba3xxxiii 85.78 (8)
Ba2vi—Ba2—Ba2vii 60.0 Ba1xxxiv—O1—Ba1xxx 90.0
O2i—Ba2—Ba3xv 148.58 (3) Ba1xxxiv—O1—Ba1xxviii 180.0
O3xi—Ba2—Ba3xv 103.8 (7) Ba1xxx—O1—Ba1xxviii 90.0
O3xii—Ba2—Ba3xv 103.8 (7) Ba1xxxiv—O1—Ba1xxix 90.0
Pd1ii—Ba2—Ba3xv 128.44 (6) Ba1xxx—O1—Ba1xxix 90.0
Pd1i—Ba2—Ba3xv 65.59 (4) Ba1xxviii—O1—Ba1xxix 90.0
Ba2xiii—Ba2—Ba3xv 149.778 (3) Ba1xxxiv—O1—Ba1xxxv 90.0 Ba2xiv—Ba2—Ba3xv 149.778 (3) Ba1xxx—O1—Ba1xxxv 90.0 Ba2vi—Ba2—Ba3xv 110.04 (2) Ba1xxviii—O1—Ba1xxxv 90.0 Ba2vii—Ba2—Ba3xv 110.04 (2) Ba1xxix—O1—Ba1xxxv 180.0
O3xi—Ba2—Ba3 103.8 (7) Ba1xxx—O1—Ba1xxxvi 180.0
O3xii—Ba2—Ba3 103.8 (7) Ba1xxviii—O1—Ba1xxxvi 90.0
Pd1ii—Ba2—Ba3 65.59 (4) Ba1xxix—O1—Ba1xxxvi 90.0
Pd1i—Ba2—Ba3 128.44 (6) Ba1xxxv—O1—Ba1xxxvi 90.0
Ba2xiii—Ba2—Ba3 110.04 (2) Ba2xxxvii—O2—Ba2xxviii 180.0 Ba2xiv—Ba2—Ba3 110.04 (2) Ba2xxxvii—O2—Ba2xxxviii 90.0 Ba2vi—Ba2—Ba3 149.778 (3) Ba2xxviii—O2—Ba2xxxviii 90.0 Ba2vii—Ba2—Ba3 149.778 (3) Ba2xxxvii—O2—Ba2xxix 90.0
Ba3xv—Ba2—Ba3 62.85 (5) Ba2xxviii—O2—Ba2xxix 90.0
O2i—Ba2—Ba1xvi 107.018 (16) Ba2xxxviii—O2—Ba2xxix 90.0
O3xi—Ba2—Ba1xvi 43.6 (7) Ba2xxxvii—O2—Ba2iv 90.0
O3xii—Ba2—Ba1xvi 152.63 (9) Ba2xxviii—O2—Ba2iv 90.0 Pd1ii—Ba2—Ba1xvi 119.33 (2) Ba2xxxviii—O2—Ba2iv 90.0
Pd1i—Ba2—Ba1xvi 65.27 (2) Ba2xxix—O2—Ba2iv 180.0
Ba2xiii—Ba2—Ba1xvi 148.818 (19) Ba2xxxvii—O2—Ba2xxx 90.0 Ba2xiv—Ba2—Ba1xvi 90.91 (2) Ba2xxviii—O2—Ba2xxx 90.0 Ba2vi—Ba2—Ba1xvi 113.456 (15) Ba2xxxviii—O2—Ba2xxx 180.0 Ba2vii—Ba2—Ba1xvi 63.792 (15) Ba2xxix—O2—Ba2xxx 90.0
Ba3xv—Ba2—Ba1xvi 60.774 (19) Ba2iv—O2—Ba2xxx 90.0
Ba3—Ba2—Ba1xvi 89.36 (2) Ba2viii—O3—Ba2xxxix 85.3 (13)
Pd2xvii—Ba3—Pd2xviii 94.07 (7) Ba2viii—O3—Ba2x 85.3 (13) Pd2xvii—Ba3—Pd2xix 94.07 (7) Ba2xxxix—O3—Ba2x 85.3 (13) Pd2xviii—Ba3—Pd2xix 94.07 (7) Ba2viii—O3—Ba1v 178.1 (17)
Pd2xvii—Ba3—Ba4 122.33 (5) Ba2xxxix—O3—Ba1v 96.02 (7)
Pd2xviii—Ba3—Ba4 122.33 (5) Ba2x—O3—Ba1v 96.02 (7)
Pd2xix—Ba3—Ba4 122.33 (5) Ba2viii—O3—Ba1vi 96.02 (7)
Pd2xvii—Ba3—Ba2 151.51 (7) Ba2xxxix—O3—Ba1vi 96.02 (7)
Pd2xviii—Ba3—Ba2 67.311 (13) Ba2x—O3—Ba1vi 178.1 (17)
Pd2xix—Ba3—Ba2 67.311 (13) Ba1v—O3—Ba1vi 82.6 (12)
Ba4—Ba3—Ba2 86.16 (3) Ba2viii—O3—Ba1 96.02 (7)
Pd2xvii—Ba3—Ba2xx 67.311 (13) Ba2xxxix—O3—Ba1 178.1 (17) Pd2xviii—Ba3—Ba2xx 67.311 (13) Ba2x—O3—Ba1 96.02 (7)
Pd2xix—Ba3—Ba2xx 151.51 (7) Ba1v—O3—Ba1 82.6 (12)
Ba4—Ba3—Ba2xx 86.16 (3) Ba1vi—O3—Ba1 82.6 (12)
Symmetry codes: (i) x, y−1/2, z−1/2; (ii) x, −y+1/2, −z+1/2; (iii) x, −y+1, −z+1; (iv) z+1/2, −x+1, −y+1/2; (v) −y+1, z, −x+1; (vi) z+1/2, x−1/2, y; (vii)
y+1/2, z, x−1/2; (viii) −y+1, z+1/2, −x+1/2; (ix) z+1, −x+1/2, −y+1/2; (x) z+1, x, y; (xi) x−1/2, −y+1/2, −z; (xii) x−1/2, y−1/2, z; (xiii) −y+1/2, z, −x+1/2; (xiv) z+1/2, −x+1/2, −y; (xv) x, −y, −z; (xvi) z, −x+1, −y; (xvii) x−1, −y+1, −z+1; (xviii) −x+1, y−1, −z+1; (xix) −x+1, −y+1, z−1; (xx) y, z, x; (xxi) z, x, y; (xxii) y, z, x−1; (xxiii) z, x−1, y; (xxiv) x−1, y, z; (xxv) −x, −y, z; (xxvi) −x+1/2, y, −z+1/2; (xxvii) −x+1/2, −y+1/2, z; (xxviii) y+1/2, z+1/2, x; (xxix) z+1/2,