Cytokine Concentrations in Men Who Have Sex with Men
Marlies Heiligenberg,a,bRené Lutter,cDasja Pajkrt,dKarin Adams,aHenry De Vries,a,e,fTitia Heijman,a,b Maarten F. Schim van der Loeff,a,bSuzanne Geerlingsb
Cluster of Infectious Diseases, Health Service Amsterdam, Amsterdam, the Netherlandsa
; Department of Internal Medicine, Division of Infectious Diseases, Center for Infection and Immunity Amsterdam (CINIMA), Academic Medical Center, Amsterdam, the Netherlandsb
; Departments of Respiratory Medicine and Experimental Immunology, Academic Medical Center, Amsterdam, the Netherlandsc
; Department of Pediatric Infectious Diseases, Academic Medical Center, Amsterdam, the Netherlandsd
; Department of Dermatology, Academic Medical Center, Amsterdam, the Netherlandse
; National Institute for Public Health and the Environment, Centre for Infectious Diseases Control, Bilthoven, the Netherlandsf
Asymptomatic
Chlamydia trachomatis
infections are common in HIV-infected men who have sex with men (MSM). Although
C.
trachomatis
combined with HIV would be likely to enhance inflammation, the asymptomatic course suggests otherwise. We
as-sessed local inflammation, mucosal damage, and cytokine concentrations in rectal mucosal fluid samples from patients with HIV
(with or without the use of combination antiretroviral therapy [cART]) and with or without the presence of rectal
C.
trachoma-tis
. Rectal swabs from 79 MSM (with and without
C. trachomatis
, HIV, and cART use) who reported a history of receptive anal
sex were analyzed for neutrophil activation (measured by myeloperoxidase [MPO]), mucosal leakage (measured by albumin and
alpha-2-macroglobulin), and proinflammatory and anti-inflammatory cytokines.
C. trachomatis
infection, HIV infection, and
cART use in MSM had no differential effects on rectal neutrophilic inflammation and mucosal damage. Interleukin 8 (IL-8) was
found to correlate with MPO, and MPO correlated with markers of mucosal damage. In HIV-negative participants, men with
C.
trachomatis
infection had lower concentrations of monocyte chemotactic protein 1 (MCP-1), IL-1
␣
, and IL-1 receptor
antago-nist (IL-1RA) than men without rectal
C. trachomatis
infection (
P
ⴝ
0.005, 0.007, and 0.07, respectively). We found no difference
in anal cytokine concentrations in HIV-infected participants in relation to the presence of
C. trachomatis
infection or cART use.
In participants with rectal
C. trachomatis
infection, those who were HIV negative had lower median concentrations of IL-8 and
IL-1
␣
than those with HIV (
P
ⴝ
0.05 and 0.06, respectively). The slope of the regression line between MPO and IL-8 was reduced
in participants with rectal
C. trachomatis
infection.
C. trachomatis
dampens cytokine concentrations but not in HIV-infected
patients. The extent of mucosal damage was comparable in all patient groups. The apparent reduced neutrophil response to IL-8
in HIV-infected patients with
C. trachomatis
infection is in accordance with its asymptomatic course.
U
rogenital infection with
Chlamydia trachomatis
is the most
commonly reported sexually transmitted infection (STI) and
continues to be a major public health problem worldwide.
Un-treated
C. trachomatis
infections can lead to serious
complica-tions, such as pelvic inflammatory disease in women and
epidid-ymitis in men (
1
). Asymptomatic
C. trachomatis
infections are
common, including in men who have sex with men (MSM) who
are infected with human immunodeficiency virus (HIV) (
2–5
).
This is thus a major clinical problem, since
C. trachomatis
tion is regarded as an important cofactor in incident HIV
infec-tions among MSM (
6
,
7
).
C. trachomatis
infection is associated
with increased HIV shedding (
8
,
9
), and the treatment of
C.
tra-chomatis
urethritis reduces seminal HIV RNA concentrations in
HIV-infected men (
10
). The exact mechanisms by which
C.
tra-chomatis
infections increase viral shedding and why
C. trachomatis
infections are often asymptomatic are not well understood. It has
been suggested that sexually transmitted infections (STIs), such as
C. trachomatis, enhance HIV transmission through an increased
release of proinflammatory cytokines (
8
), which attenuates the
local mucosal immune defense.
Several studies have assessed the concentrations of
proinflam-matory cytokines in
C. trachomatis
infections and other STIs.
In
vitro,
C. trachomatis
has increased the concentration of cytokines
in fibroblasts like synovial and epithelial cells (
11
,
12
).
Further-more,
in vivo
increases in local proinflammatory and
anti-inflam-matory cytokines in
C. trachomatis
infections have been found in
cervical lavage specimens in women (
13
) and on urethral swabs in
men (
14
,
15
). Increased concentrations of cytokines were also
de-tected in the urine of men infected with
Neisseria gonorrhoeae
before the onset of symptoms, and these concentrations peaked at
the onset of symptoms (
16
).
Taken together, it is likely that HIV-infected patients
coin-fected with
C. trachomatis
have more pronounced local
inflamma-tion than patients with either HIV or
C. trachomatis
infection. In
addition, HIV-infected patients, especially those who are not
treated with combination antiretroviral therapy (cART), may
have decreased cellular immunity (
17
), which might impede the
adaptive immune response to
C. trachomatis
and further
aggra-vate inflammation. The enhanced inflammation, however, is not
consistent with the commonly asymptomatic course of
C.
tracho-Received17 December 2012Returned for modification5 February 2013 Accepted26 July 2013
Published ahead of print31 July 2013 Address correspondence to Marlies Heiligenberg, mheiligenberg@ggd.amsterdam.nl.
Supplemental material for this article may be found athttp://dx.doi.org/10.1128
/CVI.00763-12.
Copyright © 2013, American Society for Microbiology. All Rights Reserved. doi:10.1128/CVI.00763-12
on August 17, 2020 by guest
http://cvi.asm.org/
matis
infection in HIV-infected patients. To our knowledge,
mu-cosal damage and the inflammatory and immune responses of the
rectal mucosa have not been previously studied. The aim of this
exploratory study was to assess whether rectal
C. trachomatis
in-fection status, HIV inin-fection status, and use of cART affect local
markers of mucosal damage (measured by leakage) and
inflam-mation (measured by neutrophil activation), as well as the
con-centrations of several proinflammatory and anti-inflammatory
cytokines and chemokines (interleukin 8 [IL-8], monocyte
che-motactic protein 1 [MCP-1], macrophage inflammatory protein
1

[MIP-1

], IL-1
␣
, IL-1

, interleukin 1 receptor antagonist
[IL-1RA], IL-6, and IL-10) in the rectums of MSM who have had
receptive anal intercourse.
MATERIALS AND METHODS
Study population.The outpatient STI clinic of the Public Health Service of Amsterdam (GGD Amsterdam) offers free and anonymous STI testing and treatment (18). From November 2010 to February 2011, consecutive MSM attendees of the clinic who reported having receptive anal sex in the preceding 6 months and who were visiting the clinic for an STI screening were invited to participate in the study. Further inclusion criteria were beingⱖ16 years of age and having a sufficient understanding of the study as presented in Dutch or English. Before routine consultation with the nurse, the study was explained and the patient was invited to participate. After obtaining informed consent for participation, we asked HIV-in-fected patients for written approval to obtain data on CD4 cell count, HIV RNA load, and cART use from the physician treating their HIV infection. Patients who declined to permit us to obtain data from their treating physicians were still allowed to participate. We aimed to select 90 partic-ipants from the total group who gave informed consent for cytokine test-ing. To study the effects ofC. trachomatisstatus, HIV status, and cART use on markers of inflammation, mucosal damage, and cytokine concentra-tions, we intended to recruit 15 participants in each of the following 6 groups: group A,C. trachomatis-negative and HIV-negative MSM; group B, C. trachomatis-negative HIV-infected MSM not receiving cART; group C,C. trachomatis-negative HIV-infected MSM receiving cART; group D,C. trachomatis-positive HIV-negative MSM; group E,C. tra-chomatis-positive HIV-infected MSM not receiving cART; and group F,
C. trachomatis-positive HIV-infected MSM receiving cART.
Participants who were diagnosed with lymphogranuloma venereum, rectal gonorrhea, rectal herpes, anal warts, or non-C. trachomatisproctitis were excluded. Also, participants who were diagnosed with syphilis or acute hepatitis B or C infection were excluded. For the analysis, we se-lected consecutive participants for each of the six groups. However, for groups consisting of HIV-infected MSM, we preferred those who permit-ted us to obtain data from the physician treating their HIV infection over those who did not. Also, we preferred participants without any other STI except rectalC. trachomatisinfection. The medical ethics committee of the Academic Medical Center approved the study. All participants provided written informed consent.
Procedures and laboratory testing.All patients were screened for STIs according to the clinic protocol (19). A nucleic acid amplification test (NAAT) (Gen-Probe Aptima Combo 2 assay; Gen-Probe Incorporated, San Diego, CA) was used to diagnose C. trachomatis infection. HIV screening was performed via a third-generation commercial micropar-ticle enzyme immunoassay system (AxSYM HIV Ag/Ab Combo assay; Abbott, Abbott Park, IL). HIV-positive test results were confirmed by immunoblot (INNO-LIA HIV I/II score; Innogenetics NV, Ghent, Bel-gium).
An additional anal swab for cytokine measurements was taken from all participants with a dry cotton tip swab before the standard rectal swabs for theC. trachomatisandN. gonorrhoeaetests were performed. The swab was placed 5 cm into the rectum, twisted for 10 s, and placed in 1 ml phos-phate-buffered saline (PBS) (pH 7.4) at 4°C, and debris was cleared by
centrifugation at 260⫻gfor 10 min within 24 h of collection. The super-natant was stored in aliquots at⫺20°C until analysis.
The amount of mucosal lining fluid on the swabs differed among the participants. Albumin (Alb) is a relatively small molecule in the circula-tion, which passes the mucosal barrier relatively unrestricted. Thus, the concentration in the mucosal lining fluid is almost in equilibrium with the concentration in the circulation (20) unless there is leakage across the mucosal barrier, such as that caused by inflammatory damage. Conse-quently, the concentration of albumin (Alb) in the supernatant of the swab reflects the dilution of the mucosal lining fluid. The albumin quo-tient [Q(Alb)] (calculated as [Alb]supernatant/[Alb]serum) was calculated for each participant. We adjusted for the dilution of the mucosal-lining fluid for each participant by dividing the observed values for the various pa-rameters in the swab by the Q(Alb). The amount of Alb was determined using nephelometry (modular preanalytics EVO [MPA]; Roche).
When there is substantial leakage across the rectal mucosa, Q(Alb) should not be used to adjust for the dilution of the mucosal lining fluid. To check whether there was substantial leakage, we also assessed the concentration of alpha-2-macroglobulin (A2M). A2M is a large molecule in the circulation, and its passage across the mucosal mem-brane is more restricted than that of albumin, unless there is mucosal leakage (21). This mucosal leakage can be expressed as Q(A2M) (calcu-lated as [A2M]supernatant/[A2M]serum) or by comparison of the leakage of
A2M with that of albumin in the relative coefficient of excretion (RCE) [calculated by Q(A2M)/Q(Alb) (22)]. In case of dilution of the mucosal lining fluid, the Q(Alb) and Q(A2M) are attenuated in a similar manner. In case of leakage across the mucosal barrier, the Q(A2M) values will be close to those of Q(Alb), thus raising the RCE. A2M was determined by enzyme-linked immunosorbent assay (ELISA) (21).
The concentration of myeloperoxidase (MPO) released by activated neutrophils in the supernatant was taken as a marker of neutrophilic inflammation and was measured by ELISA (lower detection limit, 1.5 ng/ml) (23). The MPO ELISA had an interassay coefficient of variation (CV) of⬍15% and an intra-assay CV of⬍15%. MPO values were ad-justed for variable dilution by dividing by the Q(Alb).
The concentrations of IL-8, MCP-1, MIP-1, IL-1␣, IL-1, IL-1RA, IL-6, and IL-10 were analyzed in the supernatant with a multiplex fluo-rescent bead assay, according to the standard protocol (Bio-Rad Labora-tories, Clinical Diagnostics Group, Hercules, CA). Fluorescence was quantified with a Bio-Plex 200 (Bio-Rad Laboratories, Clinical Diagnos-tics Group, Hercules, CA). Cytokine concentrations were adjusted for variable dilution by dividing by the Q(Alb).
Although the volume of the rectal swab samples was limited, we per-formed recoveries with standard material for A2M, MPO, and the cyto-kines IL-8, MCP-1, MIP-1, IL-6, and IL-10, which yielded recoveries between 78 and 122% or better. We were not able to perform recoveries for IL-1␣, IL-1, IL-1RA, and albumin and therefore cannot exclude that the amounts of these proteins were underestimated. The values for Alb, A2M, and MPO were based on at least two closely matching values from serial dilutions, confirming the identity of the quantified proteins. Several rectal swab samples were rerun in Luminex, yielding values very similar to those obtained before.
Statistical analyses.A value of half the lower limit of detection was assigned to samples with cytokine or MPO concentrations below the lower limit of detection of the assay. The effects ofC. trachomatisinfection status, HIV infection status, and cART use on the markers of mucosal damage and inflammation and on cytokine concentrations were analyzed by the following comparisons: (i) the effect ofC. trachomatisinfection, by comparing HIV-negative participants without and withC. trachomatis
infection (group A versus D) and by comparing HIV-infected participants without and withC. trachomatisinfection (groups B plus C versus E plus F); (ii) the effect of HIV, by comparing HIV-negative and HIV-infected participants withC. trachomatisinfection (group D versus E plus F); (iii) the effect of cART use, by comparing HIV-infected participants withC. trachomatisinfection without and with cART use (E versus F); and (iv)
on August 17, 2020 by guest
http://cvi.asm.org/
participants with self-reported anal symptoms versus participants with-out self-reported anal symptoms.
Differences between the Q(A2M), Q(Alb), RCE, MPO, and cytokine concentrations were calculated by the Wilcoxon test. No corrections for multiple testing were done. In scatter plots, fitted regression lines were calculated. We plotted Q(A2M) against Q(Alb) because a marked corre-lation would suggest no leakage across the rectal mucosa. We also plotted MPO against IL-8 forC. trachomatis-negative participants andC. tracho-matis-positive participants to assess whether there were differences in the MPO increases in reaction to IL-8 increases; IL-8 is a major chemokine for neutrophil recruitment and activation, and increases of IL-8 should cor-respond with increases in MPO.
Data analyses were performed with Stata 11.2 (Intercooled Stata; Stata, College Station, TX). Differences were considered significant at aPvalue ofⱕ0.05 and near significant atPvalues of⬎0.05 andⱕ0.1. In the figures, a log10scale was used. The adjusted values are presented inFig. 1
and2and in Tables S2, S3, and S4 in the supplemental material, unless indicated otherwise.
RESULTS
Study population.
Of 961 MSM who were screened for eligibility,
288 were excluded because they reported not having had receptive
anal sex in the 6 months preceding the study visit (see Fig. S1 in the
supplemental material). Of the 673 eligible MSM, 91 (13.5%)
re-fused to participate. Of the remaining MSM, 79 consecutive
par-ticipants were selected for groups A to F. The numbers in each
group do not total exactly 15, as too few men fit the criteria for
some groups (e.g., E and F), and some men ultimately had
differ-ent HIV and cART statuses than were initially reported (groups B
and C). The participant characteristics are depicted in
Table 1
. The
MPO, Alb, A2M, or cytokine concentrations could not be
ana-lyzed in the samples from three participants. Only five (6.3%)
MSM had self-reported rectal symptoms, such as itching or
dis-charge, and four of the five were diagnosed with rectal
C.
tracho-matis
infection.
Data on plasma HIV RNA and CD4 cell count were missing for
participants who were newly diagnosed with HIV (n
⫽
7) and for
participants who declined to give permission for obtaining
infor-mation about plasma HIV RNA and CD4 counts from the
physi-cian treating their HIV infection (n
⫽
7). One HIV-infected
par-ticipant had never received cART but nevertheless had an
undetectable viral load.
Markers of inflammation and mucosal damage (A2M,
albu-min, RCE, and MPO).
The values for the Q(A2M), Q(Alb), RCE,
and MPO concentrations for each group are shown graphically in
Fig. 1
and in Table S2 in the supplemental material.
Markers of mucosal damage.
There were no significant
differ-ences in Q(A2M), Q(Alb), and RCE between the groups (
Fig. 1
).
The relationship between Q(A2M) and Q(Alb) in sampled
muco-sal fluids is shown in Fig. S3 in the supplemental material. The
TABLE 1Demographic characteristics of 79 MSM included in the study on rectal cytokines andChlamydia trachomatisinfection, Amsterdam, 2010 to 2011aParticipant characteristics
Values by infection status, group, HIV status, and cART use
C. trachomatisnegative C. trachomatispositive
Group A, HIV⫺
Group B, HIV⫹ cART⫺
Group C, HIV⫹cART⫹
Group D,
HIV⫺ Group E, HIV⫹cART⫺
Group F, HIV⫹ cART⫹
No. 15 13 19 15 8 9
Demographics
Age (median [IQR]) (yr) 40 (27–48) 40 (33–46) 46 (42–49) 33 (29–43) 36 (30–40) 43 (29–52) Ethnicity (no. [%])
Dutch 11 (73) 11 (85) 17 (89) 11 (73) 4 (50) 7 (89)
Other 4 (27) 2 (15) 2 (10) 4 (27) 4 (50) 2 (11)
Anal symptoms (no. [%])
No 15 (100) 13 (100) 18 (95) 14 (93) 7 (88) 7 (78)
Yes 0 0 1 (5) 1 (7) 1 (13) 2 (22)
HIV clinical data
HIV plasma viral load (median [IQR]) (copies/ml)
1,930 (310–29,319)b 20 (20–20) 14,500 (10,000–254,000)c 20 (20–20)d
HIV plasma viral load (no. [%])
⬍50 copies/ml 1 (8) 19 (100) 0 7 (78)
ⱖ50 copies/ml 5 (39) 0 3 (38) 0
Data missing 7 (54) 0 5 (63) 2 (22)
CD4⫹T cells (median [IQR]) (cells/mm3)
735 (400–880)b 534 (430–690) 460 (440–940)c 670 (430–700)d
CD4⫹T cells (no. [%])
⬍200 cells/mm3 0 1 (5) 0 0
ⱖ200 cells/mm3 6 (46) 18 (95) 3 (38) 7 (78)
Data missing 7 (54) 0 5 (62) 2 (22)
aMSM, men who have sex with men; cART, combination antiretroviral therapy; IQR, interquartile range. b
Based on 6 participants with available test results.
cBased on 3 participants with available test results. d
Based on 7 participants with available test results.
on August 17, 2020 by guest
http://cvi.asm.org/
correlation between Q(A2M) and Q(Alb) indicated no mucosal
leakage, and thus adjustment by Q(Alb) for dilution of mucosal
lining fluid was justified. Only one sample deviated markedly
from the fitted line, which suggests that the mucosal barrier was
reduced for this participant.
Markers of inflammation.
Figure 1
shows the MPO
concen-trations for each group. There were no significant differences in
MPO concentrations between groups. Q(A2M) and RCE
corre-lated with MPO (not shown) (Spearman’s
⫽
0.6 and
⫽
0.5,
respectively), which is suggestive of neutrophil activation causing
mucosal damage.
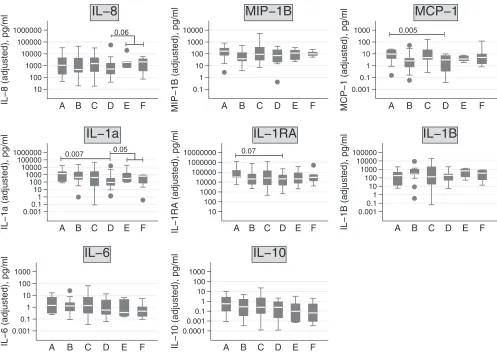
Cytokine concentrations.
Concentrations of
proinflamma-tory and anti-inflammaproinflamma-tory cytokines are shown by group in
Fig.
2
, and differences between the groups with a
P
value of
⬍0.1 are
shown in box plots. Numerical data are shown in Table S2 in the
supplemental material. Table S4 in the supplemental material
shows an overview of the differences between adjusted and
unad-justed cytokine concentrations. Although an adjustment of the
cytokine concentrations for dilution resulted in lower
P
values,
three out of the five unadjusted values already showed significant
or near-significant differences.
Influence of
C. trachomatis
on cytokine concentrations.
Among the HIV-negative participants, the median concentrations
of MCP-1, IL-1␣, and IL-1RA were unexpectedly higher (at a
P
value of
⬍
0.1) in those without rectal
C. trachomatis
infection
(group A) than in those with
C. trachomatis
infection (group D).
In HIV-infected participants, there were no differences in the
me-dian concentrations of cytokines between those with and without
rectal
C. trachomatis
infection.
Influences of HIV and cART on cytokine concentrations.
In
participants with rectal
C. trachomatis
infection, HIV-negative
MSM (group D) appeared to have lower median concentrations of
IL-8 and IL-1
␣
than participants with HIV (groups E and F)
(un-adjusted
P
⫽
0.09 and unadjusted
P
⫽
0.04, respectively). An
adjustment of the concentrations for dilution resulted in similar
differences (P
⫽
0.06 and
P
⫽
0.05, respectively). There were no
differences in median cytokine concentrations between
HIV-in-fected participants with and without cART, irrespective of the
presence or absence of
C. trachomatis
infection.
Asymptomatic versus symptomatic patients.
The median
concentrations of IL-6 and IL-10 were higher in asymptomatic
patients (n
⫽
74) than in patients with symptoms (n
⫽
5) (P
⫽
0.04 and
P
⫽
0.01, respectively) (adjusted IL-6, 0.8 versus 0.2
pg/ml, and adjusted IL-10, 0.3 versus 0.01 pg/ml, respectively).
MPO and IL-8 concentrations.
Since IL-8 is a major
chemo-kine for neutrophil recruitment and activation, we assessed the
relationship between MPO and IL-8 concentrations in men
with-out and with
C. trachomatis
infection (see Fig. S5 in the
supple-mental material), with different results. We found that increases
in IL-8 corresponded with greater increases in MPO in MSM
without
C. trachomatis
(groups A, B, and C) than in those with
C.
trachomatis
infection (groups D, E, and F). In MSM with
C.
tra-FIG 1Adjusted MPO concentrations and Q(A2M), RCE, and Q(Alb) concentrations for each group in study on rectal cytokines andC. trachomatis, Amsterdam, 2010 to 2011. For Q(A2M) and Q(Alb), the values were multiplied by 1,000. MPO, myeloperoxidase; A2M, alpha-2-macroglobulin; Q(A2M), ratio of A2M in supernatant to A2M in serum; Q(Alb), ratio of albumin in supernatant to albumin in serum. RCE was calculated by Q(A2M)/Q(Alb). MPO concentration was adjusted by dividing by the Q(Alb). The boxes represent the values from the 25 to 75 percentiles, the middle lines represent the medians, the vertical lines extend from the minimum to the maximum values, and the filled circles represent outliers.on August 17, 2020 by guest
http://cvi.asm.org/
chomatis
infection, however, more neutrophil activation was seen
at low IL-8 concentrations.
DISCUSSION
The most important finding in this study is that mucosal damage
and neutrophil inflammation were not different between patients
with or without
C. trachomatis
infection, HIV, and cART use.
Interestingly,
C. trachomatis
infection was associated with
sup-pressed cytokine (IL-8, MCP-1, IL-1
␣
, and IL-1RA) expression in
HIV-negative patients but not in those infected with HIV (IL-8
and IL-1
␣
).
C. trachomatis
infection was also associated with an
attenuated neutrophil response to IL-8, which may explain why
there were no differences in inflammation and mucosal damage
between the patient groups. To our knowledge, this is the first
report of a study on rectal cytokine concentrations and rectal
pa-rameters of inflammation. We found that rectal
C. trachomatis
infection in MSM is not paralleled by enhanced inflammation,
which may explain why rectal
C. trachomatis
infections are often
asymptomatic.
Influences of
C. trachomatis
, HIV, and cART on mucosal
damage and inflammation.
In vitro
and
in vivo
studies have
sug-gested that
C. trachomatis
infections in the urethra and vagina lead
to an increased release of inflammatory mediators (
13–15
), and
thus, we expected an increased local inflammatory response and
mucosal damage in participants with rectal
C. trachomatis
infec-tion. We used the Q(A2M), Q(Alb), and RCE [calculated by
Q(A2M)/Q(Alb)] as markers of rectal mucosal damage and MPO
concentration as a marker of the neutrophil inflammatory
re-sponse. There were, however, no significant differences for these
parameters, indicating no difference in the extent of mucosal
damage between the patient groups. The correlation between Alb
and A2M in rectal samples and the correlation between MPO and
markers of mucosal damage underscore the genuineness of these
findings. Since mechanical stress can lead to inflammation (
24
),
local mechanical stress in the rectum caused by receptive anal sex
may have overshadowed differences in the markers of mucosal
damage and inflammation between the groups.
Influence of
C. trachomatis
, HIV, and cART on cytokine
con-centration.
C. trachomatis
infection in HIV-infected MSM
nei-ther reduced nor increased cytokine concentrations. In
HIV-neg-ative MSM, however, we found reduced cytokine concentrations
in those with
C. trachomatis
infection compared to those without
C. trachomatis
infection. These results are not due to the
adjust-ment for dilution, since three out of the five unadjusted values
0.06
10 100 1000 10000 100000 1000000
IL−8 (adjusted), pg/ml A B C D E F
IL−8
0.1 1 10 100 1000 10000
MIP−1B (adjusted), pg/ml A B C D E F
MIP−1B
0.005
0.001 0.1 1 10 100 1000
MCP−1 (adjusted), pg/ml A B C D E F
MCP−1
0.007 0.05
0.001 0.1 1 10 100 1000 10000 100000 1000000
IL−1a (adjusted), pg/ml A B C D E F
IL−1a
0.07
10 100 1000 10000 100000 1000000 10000000
IL−1RA (adjusted), pg/ml A B C D E F
IL−1RA
0.001 0.1 1 10 100 1000 10000 100000
IL−1B (adjusted), pg/ml A B C D E F
IL−1B
0.001 0.1 1 10 100 1000
IL−6 (adjusted), pg/ml A B C D E F
IL−6
0.0001 0.001 0.1 1 10 100 1000
IL−10 (adjusted), pg/ml A B C D E F
IL−10
FIG 2Adjusted cytokine concentrations by group in study on rectal cytokines andC. trachomatisinfection, Amsterdam, 2010 to 2011. Cytokine concentrations were adjusted by dividing by the Q(Alb). IL, interleukin; MIP, macrophage inflammatory protein; Q(Alb), ratio of albumin in supernatant to albumin in serum. The boxes represent the values from the 25 to 75 percentiles, the middle lines represent the medians, the vertical lines extend from the minimum to the maximum values, and the filled circles represent outliers.
on August 17, 2020 by guest
http://cvi.asm.org/
already showed significant or near-significant differences. In
pre-vious studies,
C. trachomatis-infected epithelial cells were found
to release more IL-1
␣
(
12
), and
C. trachomatis
infections of the
urethra and cervix displayed increased IL-6, IL-8, and IL-10
con-centrations (
12–15
,
25
). It is probable that the microbial load of
the gastrointestinal tract is increased more than those in the cervix
and urethra (
26
), which may underlie observed differences in the
inflammatory responses to
C. trachomatis
infection between the
rectum and the cervix or urethra. An alternative explanation for
the reduced cytokine concentrations in
C. trachomatis-infected
HIV-negative patients is that
C. trachomatis
infection induces
in-doleamine 2,3-dioxygenase (IDO) activity that inhibits immune
responses (
27
). Since IDO expression is dependent on T-cell
acti-vation, a reduced T-cell response, as may occur in HIV-infected
individuals, can prevent the induction of the inhibitory effect of
IDO. The lack of difference in cytokine concentrations between
HIV-infected MSM with or without
C. trachomatis
infection in
our study supports that explanation.
In HIV-infected participants in our study with rectal
C.
tracho-matis
infection, the median cytokine concentrations did not differ
between MSM who used cART and those who did not. This lack of
difference is in contrast to the findings of a study in HIV-infected
women without
C. trachomatis
infection (
28
) that showed that the
concentrations of tumor necrosis factor alpha (TNF-␣), IL-6, and
IL-1

in cervical lavages decreased after initiation of cART. Our
results may also be explained by the absence of a difference in
immune status. In our participants with and without cART, all
had relatively high concentrations of CD4
⫹T cells; the median
circulating CD4
⫹T cell concentrations were 670 and 460 cells/
mm
3, respectively.
Asymptomatic versus symptomatic participants.
Most
C.
trachomatis-infected participants (88%) in our study were
asymp-tomatic. In another study, increased concentrations of cytokines
were detected in the urine of
N. gonorrhoeae-infected men before
the onset of symptoms, and these concentrations peaked
simulta-neously with the onset of symptoms (
16
). In our study, the lack of
symptoms in those with
C. trachomatis
infection might be
ex-plained by the fact that cytokine concentrations did not differ in
HIV-infected MSM with and without
C. trachomatis
infection and
were reduced in HIV-negative MSM infected with
C. trachomatis.
Another explanation is that the median concentrations of IL-6
and IL-10 were higher in asymptomatic participants. Since IL-6
and IL-10 can have anti-inflammatory effects, increased
concen-trations of these cytokines may lead to a decreased inflammatory
response and, therefore, fewer symptoms.
A limitation of this study is the small number of participants in
each group. Nevertheless, the study still required the screening of
nearly 1,000 men attending the STI clinic in order to include the
76 that were eventually included in the analysis. As a result, the
power of the study was limited, which means that we might have
missed important differences in the data. Since there were no data
available on cytokine concentrations in rectal fluid samples, we
performed no calculations for sample size. This was a pilot study,
and we aimed to include 15 participants per group. In view of the
dynamic range of most cytokines, strong effects on cytokine
con-centrations due to
C. trachomatis, HIV, or both would have been
detected. No corrections for multiple testing were done. Since
patients not receiving cART had relatively high CD4
⫹T-cell
counts, the contrast between HIV-infected participants receiving
cART and those not receiving cART was small. This may explain
why we did not find a difference in cytokine concentrations
be-tween these groups.
The adjustment for dilution of the samples by Q(Alb) might
have resulted in overadjustment or underadjustment of the
re-sults. However, as there were no significant differences in the
mea-sured parameters of mucosal damage and the results were
compa-rable without adjustment for dilution, overestimation or
underestimation was not expected. We did not inquire about the
most recent occurrence of receptive anal intercourse. Recent
re-ceptive anal sex might have caused local trauma, which might have
affected local cytokine release, markers of inflammation, and
mu-cosal leakage. It is possible that we did not find differences in the
inflammatory markers between the groups because all the men
may have had local trauma caused by receptive anal sex. Another
limitation is that we were unable to distinguish between truly
ac-tive
C. trachomatis
infection and the deposition of genetic material
due to recent anal sexual activity. Bacterial load measurements
might have helped to distinguish these two conditions, but some
studies suggest that bacterial load measurements may be unable to
make that distinction (
29
,
30
); we did not have bacterial load
measurements. Furthermore, we have no information about the
estimated duration of the
C. trachomatis
infections, which might
have influenced our results.
In conclusion, levels of mucosal damage and neutrophil
in-flammation did not differ between patients with or without
C.
trachomatis
infection, HIV infection, and cART use.
C.
trachoma-tis
infection was associated with suppressed cytokine (IL-8,
MCP-1, IL-1␣, and IL-1RA) expression in HIV-negative patients
but not in those infected with HIV (IL-8 and IL-1
␣
).
C.
trachoma-tis
infection was also associated with an attenuated neutrophil
response to IL-8. Thus,
C. trachomatis
infection in MSM is not
paralleled by enhanced inflammation, which may explain why
C.
trachomatis
infections are often asymptomatic. Further studies are
required to clarify the exact process by which
C. trachomatis
es-capes the mechanisms in the rectum that initiate inflammation
and eradication of a pathogen.
ACKNOWLEDGMENTS
We thank all participants and colleagues of the STI clinic of the Health Service of Amsterdam, especially Anne Rutte for the inclusion of partici-pants and sample handling and Martijn van Rooijen for data manage-ment. We thank our colleagues at the Public Health Laboratory (Health Service Amsterdam) for STI testing, Barbara Dierdorp, Tamara Dekker, and Marianne van de Pol (AMC, Departments of Experimental Immu-nology and Respiratory Medicine) for the determination of inflammatory parameters and cytokines, Ronald Geskus, Jan Prins, and Ferdinand Wit for critical review of the manuscript, and Sally Ebeling for editorial assis-tance.
The authors disclose no conflicts of interest.
H.D.V., D.P., S.G., R.L., M.H., T.H., and M.F.S.V.D.L. designed the study. M.H. and M.F.S.V.D.L. were responsible for statistical analysis. M.H., M.F.S.V.D.L., R.L., and S.G. wrote the paper. All authors assisted in revis-ing the manuscript and have seen and approved the final submitted ver-sion of the manuscript.
This study was funded by grant no. 7115 0001 from the Netherlands Organisation for Health Research and Development (ZonMw).
The funding agency had no role in the study design, data collection, data analysis, data interpretation, or the writing of the paper.
REFERENCES
1.Paavonen J, Eggert-Kruse W.1999.Chlamydia trachomatis: impact on human reproduction. Hum. Reprod. Update5:433– 447.
on August 17, 2020 by guest
http://cvi.asm.org/
2.Heiligenberg M, Rijnders B, Schim van der Loeff MF, de Vries HJ, van der Meijden WI, Geerlings SE, Fennema HS, Prins M, Prins JM.2012. High prevalence of sexually transmitted infections in HIV-infected men during routine outpatient visits in the Netherlands. Sex. Transm. Dis.
39:8 –15.
3.Kent CK, Chaw JK, Wong W, Liska S, Gibson S, Hubbard G, Klausner JD.2005. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin. Infect. Dis.41:67–74. 4.Rieg G, Lewis RJ, Miller LG, Witt MD, Guerrero M, Daar ES.2008.
Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strat-egies. AIDS Patient Care STDS22:947–954.
5.Annan NT, Sullivan AK, Nori A, Naydenova P, Alexander S, McKenna A, Azadian B, Mandalia S, Rossi M, Ward H, Nwokolo N.2009. Rectal chlamydia–a reservoir of undiagnosed infection in men who have sex with men. Sex. Transm. Infect.85:176 –179.
6.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, Zimba D, Vernazza PL, Maida M, Fiscus SA, Eron JJ, Jr.1997. Reduc-tion of concentraReduc-tion of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet349:1868 –1873.
7.Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, MacDonald K, Walmsley S, Rebbapragada A.2008. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J. Reprod. Immunol.77:32– 40.
8.Johnson LF, Lewis DA.2008. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex. Transm. Dis.35:946 –959.
9.Rotchford K, Strum AW, Wilkinson D.2000. Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: systematic review and data synthesis. Sex. Transm. Dis.27:243–248. 10. Sadiq ST, Taylor S, Kaye S, Bennett J, Johnstone R, Byrne P, Copas AJ,
Drake SM, Pillay D, Weller I.2002. The effects of antiretroviral therapy on HIV-1 RNA loads in seminal plasma in HIV-positive patients with and without urethritis. AIDS16:219 –225.
11. Hanada H, Ikeda-Dantsuji Y, Naito M, Nagayama A.2003. Infection of human fibroblast-like synovial cells withChlamydia trachomatis re-sults in persistent infection and interleukin-6 production. Microb. Pathog.34:57– 63.
12. Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, Fierer J, Stephens RS, Kagnoff MF.1997. Secretion of proinflammatory cytokines by epithelial cells in response toChlamydiainfection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Invest.
99:77– 87.
13. Vats V, Agrawal T, Salhan S, Mittal A.2007. Primary and secondary immune responses of mucosal and peripheral lymphocytes during Chla-mydia trachomatisinfection. FEMS Immunol. Med. Microbiol.49:280 – 287.
14. Pate MS, Hedges SR, Sibley DA, Russell MW, Hook EW, III, Mestecky J.2001. Urethral cytokine and immune responses inChlamydia tracho-matis-infected males. Infect. Immun.69:7178 –7181.
15. Guha D, Chatterjee R.2009. Cytokine levels in HIV infected and unin-fected Indian women: correlation with other STAs. Exp. Mol. Pathol.86:
65– 68.
16. Ramsey KH, Schneider H, Cross AS, Boslego JW, Hoover DL, Staley TL, Kuschner RA, Deal CD.1995. Inflammatory cytokines produced in response to experimental human gonorrhea. J. Infect. Dis.172:186 –191.
17. Alimonti JB, Koesters SA, Kimani J, Matu L, Wachihi C, Plummer FA, Fowke KR.2005. CD4⫹T cell responses in HIV-exposed seronegative women are qualitatively distinct from those in HIV-infected women. J. Infect. Dis.191:20 –24.
18. Van Rooijen MS, van Leeuwen AP. 2010. Jaarverslag 2010. Soa-polikliniek, Amsterdam, The Netherlands.
19. Heijman TL, Van der Bij AK, De Vries HJ, Van Leent EJ, Thiesbrum-mel HF, Fennema HS. 2007. Effectiveness of a risk-based visitor-prioritizing system at a sexually transmitted infection outpatient clinic. Sex. Transm. Dis.34:508 –512.
20. Thompson AB, Mueller MB, Heires AJ, Bohling TL, Daughton D, Yancey SW, Sykes RS, Rennard SI.1992. Aerosolized beclomethasone in chronic bronchitis. Improved pulmonary function and diminished airway inflammation. Am. Rev. Respir. Dis.146:389 –395.
21. Out TA, Jansen HM, van Steenwijk RP, de Nooijer MJ, van de Graaf EA, Zuijderhoudt FM. 1987. ELISA of ceruloplasmin and alpha-2-macroglobulin in paired bronchoalveolar lavage fluid and serum samples. Clin. Chim. Acta165:277–288.
22. Schoonbrood DF, Lutter R, Habets FJ, Roos CM, Jansen HM, Out TA.
1994. Analysis of plasma-protein leakage and local secretion in sputum from patients with asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med.150:1519 –1527.
23. Bresser P, Out TA, van Alphen L, Jansen HM, Lutter R.2000. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronicHaemophilus influenzaeairway infection. Comparison with non-infected patients with chronic obstructive pulmonary disease. Am. J. Re-spir. Crit. Care Med.162:947–952.
24. Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, van der Poll T, Vroom MB, Hollmann M, Schultz MJ.2008. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology108:46 –54.
25. Al-Mously N, Eley A.2007. Interaction ofChlamydia trachomatisserovar E with male genital tract epithelium results in secretion of proinflamma-tory cytokines. J. Med. Microbiol.56:1025–1032.
26. Vossenkämper A, Macdonald TT, Marchès O.2011. Always one step ahead: how pathogenic bacteria use the type III secretion system to ma-nipulate the intestinal mucosal immune system. J. Inflamm. (Lond.)8:11. doi:10.1186/1476-9255-8-11.
27. Ibana JA, Belland RJ, Zea AH, Schust DJ, Nagamatsu T, AbdelRahman YM, Tate DJ, Beatty WL, Aiyar AA, Quayle AJ.2011. Inhibition of indoleamine 2,3-dioxygenase activity by levo-1-methyl tryptophan blocks gamma interferon-inducedChlamydia trachomatispersistence in human epithelial cells. Infect. Immun.79:4425– 4437.
28. Sachdeva RK, Wanchu A, Bagga R, Malla N, Sharma M.2010. Effect of non-nucleoside reverse transcriptase inhibitors on cytokine, chemokine, and immunoglobulin profiles in serum and genital secretions of HIV-infected women. J. Interferon Cytokine Res.30:299 –310.
29. Wiggins R, Graf S, Low N, Horner PJ, Chlamydia Screening Studies (ClaSS) Study Group.2009. Real-time quantitative PCR to determine chlamydial load in men and women in a community setting. J. Clin. Mi-crobiol.47:1824 –1829.
30. Gomes JP, Borrego MJ, Atik B, Santo I, Azevedo J, Brito de Sá A, Nogueira P, Dean D.2006. CorrelatingChlamydia trachomatisinfectious load with urogenital ecological success and disease pathogenesis. Mi-crobes Infect.8:16 –26.